1. Background
Until the mid-19th century, when synthetic dyes became prevalent, all dyes were derived from natural sources. Before their application in textile dyeing, natural dyes were used for body painting, cosmetics, and coloring handicrafts such as pottery and baskets. Over 15,000 years ago, our ancestors utilized natural pigments to decorate cave walls.
Significant advancements in dyes, chemicals, and dyeing processes have occurred within the textile industry. However, this industry has a substantial environmental impact, particularly in terms of water and soil pollution. During the dyeing process, chemicals are released into the environment, contaminating water and soil, as well as emitting harmful gases into the atmosphere. Many of the dyes and chemicals used in these processes are toxic and non-biodegradable, leading to adverse changes in the natural environment.
These disruptions to natural resources contribute to altered air flows, increased heat, and elevated atmospheric temperatures. The concept of a green or environmentally friendly approach is not new in the textile industry; it aligns with the broader goal of environmental protection. Science and technology have made significant efforts to develop eco-friendly processes aimed at minimizing environmental harm.
Recently, there has been renewed interest in using natural dyes in textile dyeing. This resurgence is driven by stringent regulations imposed by many countries in response to the allergic and toxic effects associated with synthetic dyes. It is widely believed that natural dyes are more environmentally friendly than synthetic alternatives. Natural dyes are characterized by better biodegradability and a reduced environmental footprint.
Hibiscus sabdariffa L., belonging to the Malvaceae family, is a small tropical annual shrub native to Africa and widely distributed in Southeast Asia and Central America (1, 2). Commonly referred to as hibiscus, H. sabdariffa is internationally recognized for its diverse uses. Many parts of the plant are utilized and prescribed in traditional medicine in countries such as those in Africa, India, Mexico, Brazil, China, and Iran (3).
The leaves, consumed as a vegetable, exhibit diuretic, antiseptic, digestive, purgative, sedative, and astringent properties (4). The calyces are traditionally used to manage high blood pressure, treat liver disorders, and serve as a diuretic, digestive aid, and sedative (5, 6). Compared to other parts of the plant, the seeds are less frequently mentioned in traditional medicine. However, they are roasted and consumed as food and are traditionally used as a demulcent, diuretic, laxative, and tonic (3).
2. Methods
2.1. Steps for Conducting Experiments
2.1.1. Washing Silk Items
To prepare silk goods for dyeing, it is necessary to remove waste materials such as grease, wax, thorns, shavings, and dirt. For this purpose, 2 g/L of non-ionic textile soap was used to wash the silk yarn.
The silk yarns were soaked and washed in a bath containing a solution of 2 g/L of detergent at a liquor ratio (L:R) of 1:50 for 30 minutes at a temperature of 60°C. The items were introduced into the bath at an initial temperature of 30°C, which was gradually raised to 60°C, and the washing process was carried out for 30 minutes at this temperature.
2.2. How to Extract
To extract the dye from the sour tea plant, 50 g of the plant material were boiled in 1 L of water until the volume was reduced by 50%. To maintain consistent dyeing conditions in both the extract and dry dye states, and due to the presence of natural acids in the plant, the use of any chemicals during the extraction process was avoided.
2.3. Dyeing Method
Silk goods were dyed using two methods: Prechrome and metachrome, in both dry dye and extract dye forms. To preserve the standard conditions and account for the sensitivity of silk fiber, low-concentration acid and near-neutral conditions (pH 5) with a mordant concentration of 7% were used. The amount of dye for all samples was standardized:
- For the dry dye method: Thirty percent of the dry dye by weight.
- For the extract dye method: Thirty percent of the bath volume, equivalent to 150 g/L of extract, was used.
In the prechrome dyeing process, the silk samples were dyed in a dye bath with a L:R of 1:50 for 45 minutes. The dyestuff was introduced into the dye bath at a L:R of 1:40 at an initial temperature of 40°C and left for 10 minutes. The silk samples were then introduced into the bath at the same temperature (40°C) and gradually heated to boiling over 30 minutes. The dyeing process was continued at boiling temperature for 60 minutes.
3. Results
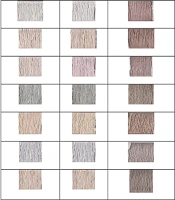
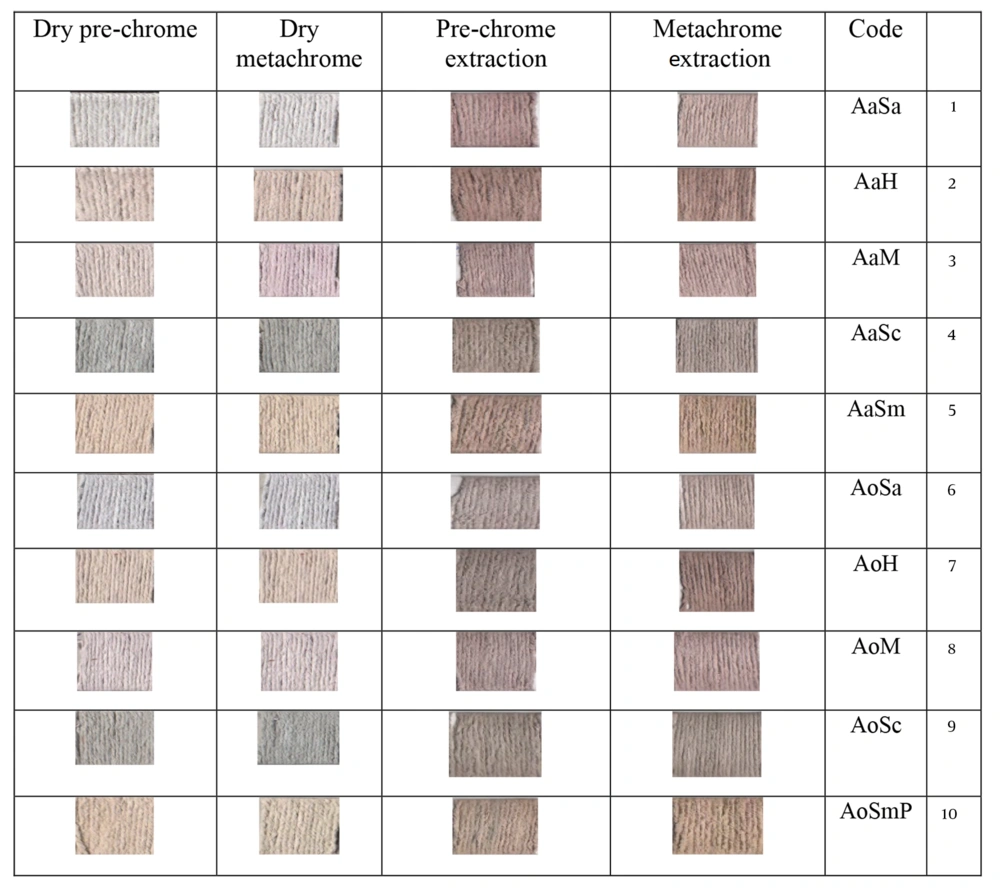
3.1. Dyed Samples
Figure 1 illustrates the samples dyed using the extraction method and the dry method. As evident from the Table 1, the samples dyed with the dye extract appear darker than those dyed with the dry dye. Notably, in the samples dyed using the dry dye under the prechrome and metachrome A methods, half of the samples displayed negative A values. This indicates that some samples in this method tended toward a green hue. In contrast, none of the samples dyed using the extraction method exhibited negative A values. It is important to note that the negative A values in the dry method were associated with samples treated with aluminum sulfate and iron sulfate.
| Bacterial Strain | Concentration 50 mg/mL | Concentration 100 mg/mL |
|---|---|---|
| Staphylococcus aureus | 6 | 10 |
| Streptococcus pyogenes | 3.4 | 7 |
| Streptococcus pneumoniae | 5 | 8 |
| Enterococcus faecalis | 4 | 6 |
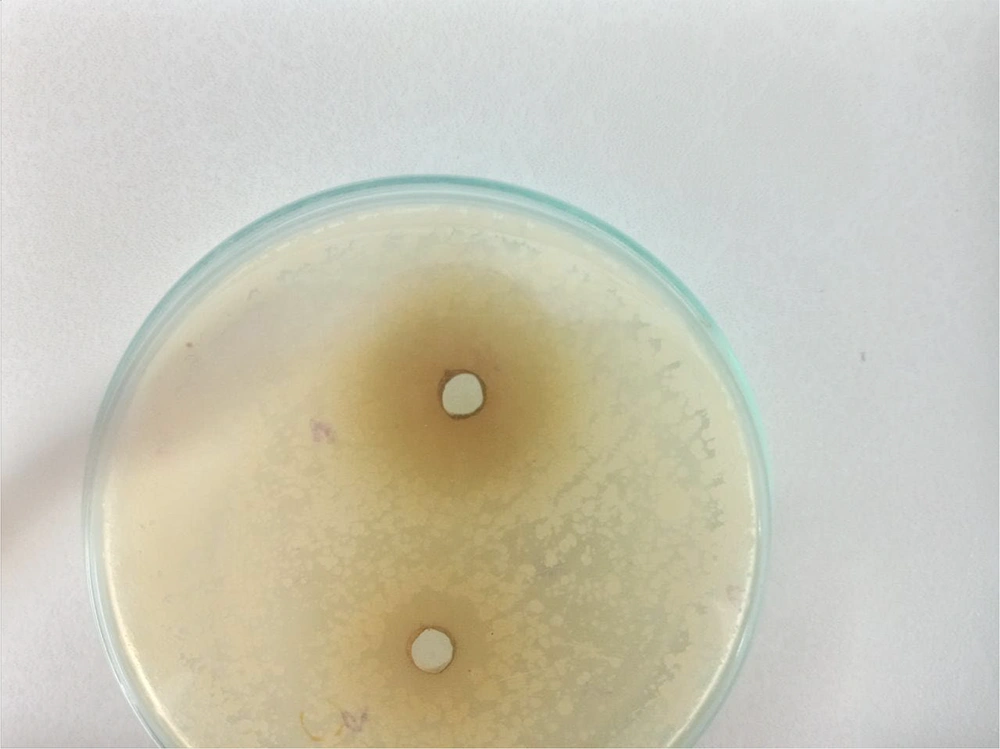
3.2. Antibacterial Results of the Medicinal Plant Sour Tea on Standard Bacteria
This study demonstrated that the diameter of the inhibitory zone of sour tea extract at a concentration of 50 mg/mL was largest against Staphylococcus aureus (6 mm) and smallest against Enterococcus faecalis (4 mm). Furthermore, at a concentration of 100 mg/mL, the sour tea extract exhibited the largest inhibitory zone diameter against S. aureus (10 mm), as shown in Table 1 and Figure 2.
4. Discussion
In general, the results of the color difference analysis of the samples indicated that the greatest color difference was associated with the dry dye. In this type of dyeing, both in the metachrome and prechrome methods, the oxalic acid variable resulted in a greater color difference compared to acetic acid. Conversely, in the dyeing process using the dye extract method, acetic acid produced a greater color difference.
For dyeing with the extract of the toothed thorn, the greatest color difference was observed in both the prechrome and metachrome methods. Meanwhile, in the samples dyed with dry dyes under the prechrome and metachrome methods, half of the samples exhibited negative A values, indicating a tendency toward a green hue. In contrast, none of the samples dyed using the extraction method showed negative A values. It is noteworthy that the negative A values in these samples were related to those dyed with aluminum sulfate and iron sulfate.
In another study that synthesized nanoparticles using sour tea extract and examined their antimicrobial activity, the results revealed strong inhibitory effects against bacteria. Specifically, the nanoparticles demonstrated high efficacy against Escherichia coli, S. aureus, S. intermedius, Proteus mirabilis, and Pseudomonas aeruginosa, with inhibitory concentration (IC50) values ranging from 0.05 ± 0.15 to 4.64 ± 0.05 mg/mL (7).
In another study, the results indicated that the crude metabolites of the methanolic extract of H. sabdariffa (flowers) demonstrated relatively significant antimicrobial activity against gram-positive pathogens. Specifically, the diameter of the inhibition zone against S. aureus was 13 mm at a concentration of 5 mg/mL, while the inhibition zone against E. coli at the same concentration was 11 mm (8).
Abass et al. investigated the antimicrobial effect of Red Roselle (H. sabdariffa) against various types of oral bacteria. The study revealed that at a concentration of 100 mg, the diameter of the inhibition zone against Streptococcus mutans was 27.6 mm, against S. aureus was 22.3 mm, and against E. faecalis was 22.4 mm (9).
In another study, the effect of the aqueous extract of H. sabdariffa on E. coli was evaluated. Using a standard microdilution assay, the extract showed a minimum inhibitory concentration (MIC) of 6.5 mg/mL against the growth of enteropathogenic E. coli (EPEC). A time-kill kinetics assay demonstrated significant bactericidal activity at 24 hours with a concentration of 25 mg/mL. Furthermore, the extract effectively prevented basal induction (10).
Another study on the antibacterial properties of the methanolic extract of H. sabdariffa calyces revealed the presence of effective antibacterial agents. These agents exhibited significant inhibition zones against both gram-negative and gram-positive bacteria tested, surpassing the effects of penicillin, which showed weak or no activity, and competing with gentamicin (11).
A comparative study examined the antimicrobial effects of an aqueous extract of red rose calyx (RE), chlorhexidine (CH), amoxicillin-clavulanic acid (ACA), tetracycline (Tet), and metronidazole (Met) on S. mutans, S. aureus, and E. faecalis. The results showed that at a dilution of 25 mg/mL, the inhibition zone diameters for S. mutans, S. aureus, and E. faecalis were 9.1 mm, 7.5 mm, and 8 mm, respectively (9).
In another study, the antimicrobial activity of sour tea extract was tested against S. mitis and S. oralis. The extract inhibited 29.1% of S. mitis and 63.23% of S. oralis bacteria (12).
Finally, the study by Marquez-Rodriguez et al. investigated the in vitro antibacterial activity of sour tea phenolic extract and its potential application in beef preservation. The results showed that the MICs against E. coli, Salmonella enterica, S. aureus, Listeria monocytogenes, and Bacillus cereus were 300, 300, 200, 200, and 200 mg/mL, respectively (13).
4.1. Conclusions
The results demonstrated that the sour tea plant possesses significant antimicrobial and color-producing properties.