1. Background
Over time, various tools have been developed to study human movement. One such tool is transcranial magnetic stimulation (TMS), which is utilized in both therapeutic settings and research. The TMS is a non-invasive technique that allows for the assessment of nerve projections to the descending pathways of the nervous system during both static (1) and dynamic movements (2). The primary variable derived from TMS is the motor-evoked potential (MEP), which reflects the integrity of the corticospinal pathway (3, 4). A major challenge in utilizing MEP as an indicator of primary motor cortex (M1) excitability is its susceptibility to changes in both cortical and subcortical circuits (5-7). Variations in human neural circuits due to anatomical and physiological differences can affect MEP values, influenced by factors such as muscle contraction type, synaptic function, previous muscle activity, mental imagery, and the co-contraction (MCo) or co-activation (MCa) of agonist and antagonist muscles (4, 8-10).
The MCo or MCa, referring to the simultaneous activation of agonist and antagonist muscles, occurs to varying degrees during all contractions (11). The MCo and MCa are often used interchangeably to describe agonist-antagonist MCo around a joint (12). This MCo can enhance strength (3), improve joint stability (13), and boost muscle performance (14). The neurological regulation of agonist and antagonist muscles is organized in a reciprocal manner, where activation of the agonist is typically coupled with the inhibition of the antagonist.
Multiple mechanisms have been proposed to explain this phenomenon at both cortical and subcortical levels. Research at the spinal or subcortical level suggests that their contribution to MCa is minimal. For instance, spinal reflexes usually produce reciprocal effects in the activation of agonist and antagonist muscles or other muscle groups (15), with such reflexive actions typically confined to a single muscle group. In contrast, other studies highlight a more substantial role for the motor cortex in the MCa phenomenon (16). Research has identified two distinct neuronal populations within the M1 of nonhuman primates. Activation of one population induced reciprocal changes in the activity of agonist-antagonist muscle pairs, whereas activation of the other caused parallel changes, thereby regulating their MCo levels. Most studies on cortical neuron activation have predominantly demonstrated reciprocal effects, where the activation of one muscle group is accompanied by either no change or suppression of the antagonist muscle's activity (17).
Conversely, studies utilizing TMS have revealed both reciprocal and nonreciprocal effects on corticospinal excitability (18). For example, Neige et al. found that differences in corticospinal excitability between flexors and elbow extensors are related to modulatory patterns that are not necessarily reciprocal (18). Thus, it appears that the MCo of both agonist and antagonist muscles affects the excitability of the corticospinal pathway. Kesar et al. demonstrated that the MCa of both agonist and ankle antagonist muscles enhances corticospinal excitability (9). Geertsen et al. found that voluntary contractions in the ankle muscle are linked to prior facilitation in the antagonist muscle, likely modulated by subcortical circuits (5). The activation of synergist (19) and antagonist muscles (9) also alters the excitability of supraspinal and spinal segmental circuits, thereby modulating TMS-evoked MEP responses in the target muscle.
From a practical perspective, implementing MCa or MCo of agonist and antagonist muscles during exercise can complicate the task. For instance, in resistance training, individuals enhance the activity of their arm muscles by rotating the forearm against a weight (as in movements like forearm curls with a dumbbell) (20). Functionally, Arai et al. showed that MCo of agonist and antagonist muscles enhanced jump performance compared to isolated muscle contractions (14). Consequently, it seems that MCo as a technique may have a more significant impact on the nervous system.
Understanding the influence of the antagonist muscle's activation state on TMS-evoked MEP amplitude can provide valuable insights for interpreting and designing TMS studies. While MCo has been widely examined in lower limbs, particularly for gait and posture, fewer studies have investigated upper-limb corticospinal excitability during MCo. Existing research on arm muscles shows conflicting results, with some studies reporting reciprocal inhibition (17) and others demonstrating MCa-driven facilitation (18). These discrepancies may arise from task-specific demands (e.g., precision vs. force tasks) or methodological differences in assessing corticospinal pathways. This study significantly advances existing knowledge by specifically investigating corticospinal excitability during agonist-antagonist MCo in upper-limb muscles, an understudied area compared to extensive lower-limb research. Unlike previous studies that reported inconsistent findings (reciprocal inhibition vs. MCa facilitation) in arm muscles, we employ a standardized MCo protocol to resolve these discrepancies. Furthermore, we bridge the gap between neurophysiological mechanisms (TMS-evoked MEP modulation) and functional outcomes, offering potential applications in rehabilitation and training. Our rigorous task design and focus on upper-limb specificity provide novel insights into whether MCo effects generalize across limbs or exhibit distinct neural control patterns.
2. Objectives
The present study will investigate the effect of MCo of agonist and antagonist muscles on corticospinal excitability in arm muscles.
3. Methods
3.1. Participants
Participants were recruited through multiple approaches, including institutional bulletin board advertisements, targeted outreach to research volunteers in an institutional database, and participant referrals. Eligibility criteria required: (A) availability for all study sessions, (B) no current use of neuroplasticity-affecting medications (e.g., antidepressants), (C) no TMS contraindications (e.g., epilepsy history), and (D) no recent strength training (past 12 months) or neuromuscular disorders. Potential participants completed initial phone screenings followed by in-person evaluations to confirm eligibility, ensuring participant safety and controlling for confounding variables while maintaining sample homogeneity. Seventeen participants (17 men, aged 28.3 ± 2.2 years; 70.9 ± 8.5 kg) volunteered to participate.
3.2. Research Procedure
This study is a randomized, controlled, within-subject (repeated measures) crossover design following CONSORT guidelines for clinical trials (17). Participants performed the present research protocol in four separate sessions. Participants were called to the laboratory during five sessions. A familiarization session (first session) was held for participants 24 hours before the initial assessment of research variables. In subsequent sessions, participants performed four TMS modes randomly (computer-generated random sampling) with a controlled balance design, maintaining at least a one-day interval between sessions to minimize carryover effects.
3.3. Measurement Procedure
3.3.1. Maximal Voluntary Contraction and Max of Electromyography
Following a warm-up, maximal voluntary contraction (MVC) measurements for the biceps brachii (BB) and triceps brachii (TB) were conducted with the shoulder and elbow positioned at 90°. Manual resistance was applied using a break technique, and the MVC was measured with a MicroFet 2 hand-held dynamometer (Hogan Health Industries, Inc., 8020 South 1300 West, West Jordan, USA). The participant's forearm was kept in a neutral position (21), and any gravitational effects were mitigated by having the upper limb supported. Isometric muscle force from elbow flexion and extension was measured, and the maximal value from a 3-second peak contraction during three attempts was defined as the MVC EMG. This maximal EMG value was used to set a low-level target activation background (5% MVC EMG), which participants maintained during TMS assessments and also for normalizing the MEP.
3.3.2. Surface Electromyography
Before electrode placement, the area was shaved to remove fine hair, then treated with an abrasive skin gel to eliminate dead skin, and cleaned with 70% isopropyl alcohol wipes. Surface electromyography (sEMG) was recorded from the BB and TB muscles using bipolar Ag-AgCl electrodes. For the BB, the measurement site was determined by marking the skin two-thirds of the distance between the acromion and the lateral epicondyle while the participant stood in a relaxed anatomical position (22). This mark was extended to the most anterior point of the muscle, where the electrodes were placed 2 cm apart over the mid-belly of the BB, with a ground electrode positioned on the lateral epicondyle of the humerus (23). For the TB, the proximal electrode was placed approximately halfway between the humeral head and the olecranon process, while the distal electrode was positioned over the distal tendon. The sEMG signals were amplified (x1000), bandpass filtered (20 Hz - 1 kHz), digitized online at 2 kHz, recorded with an ME6000 device, and analyzed using MegaWin software (v.3.1).
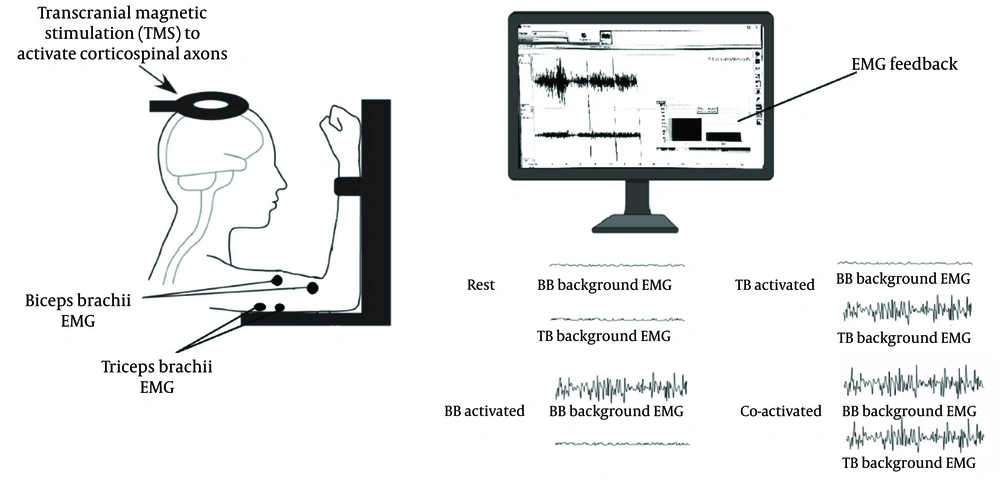
3.3.3. Electromyography Feedback for Maintaining Consistent Background Electromyography Activation
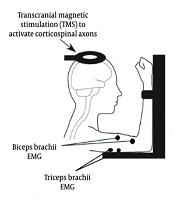
The raw EMG signals from the BB and TB muscles were recorded using the ME6000 device and processed through a custom EMG feedback program (MegaWin software, v.3.1). This program computed the root mean square (RMS) EMG for both muscles and provided participants with a real-time visual representation of their RMS EMG activity. Prior to testing, participants practiced simultaneous activation of the BB and TB while receiving real-time visual biofeedback of both muscles' EMG activity. During testing conditions that required activation of a single muscle (e.g., BB activation) or MCo, participants were provided with EMG feedback to ensure they maintained the muscle’s activation within the target EMG range (5% MVC EMG) (Figure 1).
Schematic representation of the experimental setup. Key components include surface electromyography (sEMG) electrodes placed on the tibialis anterior and soleus muscles, and a transcranial magnetic stimulation (TMS) coil (Magstim Rapid 2 stimulator) positioned over the primary motor cortex (M1). The diagram illustrates participant seating posture and real-time feedback alignment during co-contraction (MCo) trials. The sEMG signals were amplified (x1000), bandpass filtered (20 Hz - 1 kHz), and digitized online.
3.4. Transcranial Magnetic Stimulation
The TMS was administered using a Magstim Rapid 2 stimulator (Magstim Co., UK) equipped with a 70-mm figure-of-eight coil to generate MEPs in the actively engaged BB and TB muscles. The motor hotspot for the BB was identified by directing the current flow from posterior to anterior in the cortex. To maintain consistent stimulation at the optimal motor hotspot during testing, participants wore a snug-fitting cap marked with a latitude-longitude grid, which was aligned with the nasion–inion and interaural reference lines.
All stimuli were delivered during a low-level isometric contraction (5% RMS EMGMax during MVC) of both the BB and TB, with the exception of rest periods. For the MEPs recorded from the BB, participants maintained an elbow joint angle of 90 degrees while keeping the BB active. During the TMS trials, participants were provided with visual feedback showing an upper limit of 5% RMS EMG and were instructed to maintain their muscle activation below this threshold to ensure consistent background activity between TMS pulses. The RMS EMG was measured during the 100 ms preceding each TMS pulse. For the MEPs recorded from the TB, participants maintained a 90-degree elbow joint angle while actively engaging the TB. This level of background sEMG has been demonstrated in prior studies to produce consistent and reliable MEP amplitudes (9, 24, 25).
The order of testing for constructing corticospinal excitability recruitment curves was randomized between the BB and TB. The TMS pulses were delivered at stimulation intensities of 100, 120, 140, 160, and 180% of the resting motor threshold (RMT) (26). A total of ten stimulation pulses were delivered at each intensity, resulting in 50 pulses overall. The RMT was defined as the minimum stimulation intensity required to elicit a discernible MEP in the target muscles, with an amplitude exceeding 50 µV in five out of ten trials. Once the RMT was established, the average of the ten MEPs was recorded at this new intensity during the rest position. The stimulation intensity began at approximately 30% of the maximal stimulator output (MSO) and was gradually increased until the RMT was identified (27).
The peak-to-peak MEP amplitude was calculated to quantify corticospinal excitability during each TMS testing session, with the ten peak-to-peak MEP amplitudes averaged for each stimulation intensity and normalized against EMGmax. Motor-evoked potential amplitudes were analyzed using MegaWin software (v.3.1), which automatically flagged each stimulus with a cursor to provide peak-to-peak values in μV for both the BB and TB. Additionally, the total area under the recruitment curve (AURC) was calculated using trapezoidal integration based on data collected during the construction of corticospinal excitability and inhibition recruitment curves for both the BB and TB separately. A total of 10 single-pulse stimuli were delivered at 100 - 180% of the RMT for both muscles, and the AURC data were presented in arbitrary units (AU) (28).
3.5. Ethical Considerations
The data reported were collected as part of a larger study investigating the effect of training and detraining on cortical excitability. This study was approved by the Kharazmi University Ethics Committee (IR.KHU.REC.1399.039). All participants provided written informed consent and could withdraw at any time. Data anonymity and safety protocols for TMS interventions were strictly followed, per Helsinki Declaration guidelines.
3.6. Data Management and Analysis
All data were tested for normality using the Shapiro-Wilk test and were confirmed to follow a normal distribution (all P > 0.05), ensuring that the assumptions required for ANOVA were satisfied. A repeated measures ANOVA test was used to evaluate the total AURC variables and pre-stimulus EMG in all conditions. The Bonferroni test was used for multiple comparisons. Effect sizes (ES) were determined using Kendall’s coefficient of concordance, and the magnitude of mean differences was interpreted based on standardized effect sizes. Cohen’s d thresholds were defined as: ≤ 0.20 for a "small" effect, 0.21 - 0.80 for a "medium" effect, and > 0.80 for a "large" effect. Statistical analyses were performed using SPSS 22 for Windows, with the significance level set at P < 0.05 for all tests. Data are reported as mean ± standard deviation (SD).
The TMS-evoked MEPs were recorded under four randomized testing conditions:
1. Rest: Both the BB and TB were at rest.
2. The BB Activated: The BB was active while the TB remained at rest.
3. The TB Activated: The TB was active while the BB remained at rest.
4. The MCo: Both the BB and TB muscles were simultaneously activated.
In all testing conditions, TMS was applied with the coil positioned over the hotspot of the right BB, determined at the beginning of the session, and MEPs were recorded simultaneously from both the BB and TB. For the purpose of this study, the agonist muscle refers to the muscle targeted during TMS, from which MEPs were analyzed as the dependent variable. When analyzing TMS-evoked MEPs from the BB, the BB was considered the agonist and the TB the antagonist. Conversely, when evaluating MEPs from the TB, the TB was considered the agonist and the BB the antagonist. During conditions requiring BB or TB activation, participants received visual biofeedback to monitor the EMG activity of the muscle being activated.
Data from these four conditions were used to make four comparisons for each muscle (BB and TB) and to test the following four hypotheses listed below:
1. Comparison between rest and agonist activation
2. Comparison between rest and agonist-antagonist MCo
3. Comparison between rest and antagonist activation
4. Comparison between agonist activation and MCo
4. Results
Seventeen healthy participants (aged 20 - 27 years) voluntarily took part in the study. The results demonstrated that MCo of agonist and antagonist muscles significantly enhanced both the MEP AURC and pre-stimulus EMG activity compared to other conditions (P < 0.005). Additionally, antagonist muscle activation alone also increased the MEP AURC and pre-stimulus EMG levels relative to the agonist muscle's resting state (P < 0.005).
4.1. Total Area Under the Recruitment Curve and Pre-stimulus Electromyography For the Biceps Brachii and Triceps Brachii Muscle
The results indicated a significant main effect of testing condition on both BB (F (3,48) = 174.72; P < 0.001, η² = 0.931) and TB Total AURC (F (3,48) = 24.27; P < 0.001, η² = 0.651). Also, a significant main effect of testing condition on both BB (F (3,48) = 616.02; P < 0.001, η² = 0.975) and TB (F (3,48) = 388.88; P < 0.001, η² = 0.960) Pre-stimulus EMG. The results for the post hoc pairwise comparisons are listed for each of the comparisons below
4.1.1. Comparison Between Rest and Agonist Activation
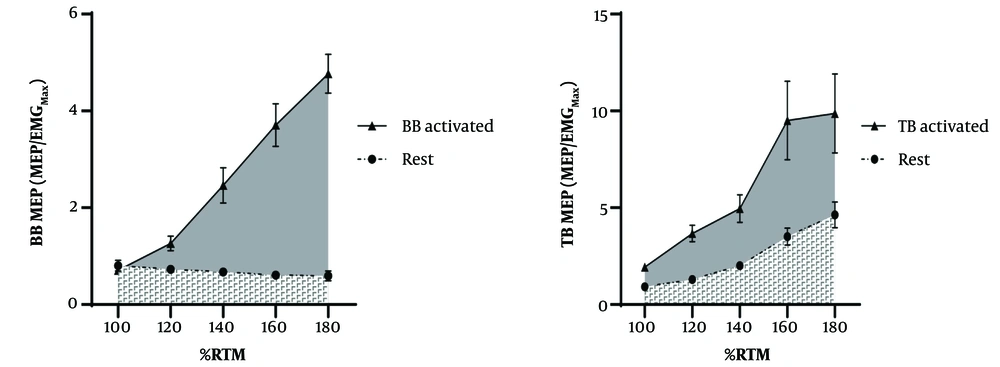
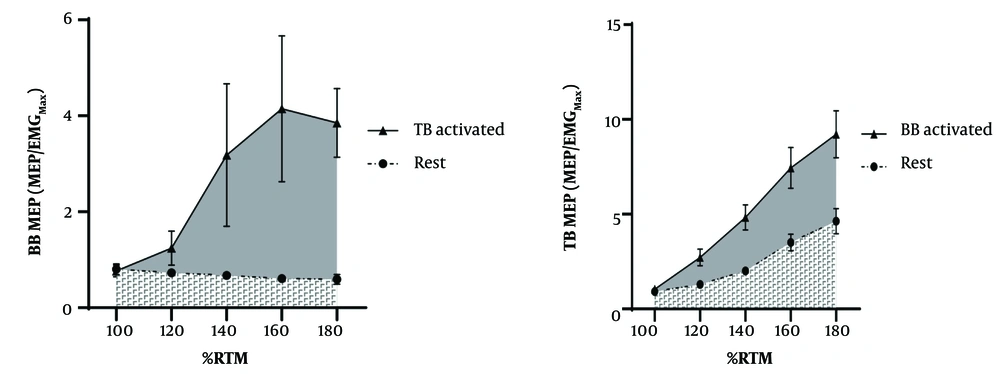
Pre-stimulus RMS amplitudes varied significantly across conditions (all P < 0.001), with resting values consistently lower than active states (d < -0.98; Table 1). Similarly, the background EMG RMS for the TB was significantly lower (d: -4.76, P < 0.001) during rest (0.0023 ± 0.0006 mV) compared to Agonist activation (0.0091 ± 0.0006 mV). Total AURC for the BB MEP was significantly larger (d: -1.75, P < 0.001) during Agonist activation (60.20 ± 2.96 Au) compared to rest (40.17 ± 2.05 Au). Also, a large effect was observed for TB AURC differences between Rest (64.44 ± 5.03 Au) and Agonist Activation (101.00 ± 6.41 Au) (d: -1.18, P = 0.004) (Figure 2).
| Muscle | Rest | Agonist | (vs. Rest) | Antagonist | (vs. Rest) | Co-activation | (vs. Rest) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| d | P-Value | d | P-Value | d | P-Value | |||||
| BB | 0.0024 ± 0.0005 | 0.022 ± 0.0026 | -1.39 | < 0.001 | 0.015 ± 0.0018 | -1.12 | < 0.001 | 0.018 ± 0.0021 | -1.3 | < 0.001 |
| TB | 0.0018 ± 0.0003 | 0.019 ± 0.0021 | -1.25 | < 0.001 | 0.012 ± 0.0015 | -0.98 | < 0.001 | 0.016 ± 0.0019 | -1.18 | < 0.001 |
Pre-stimulus Root Mean Square Amplitudes (mV) Across Experimental Conditions with Statistical Comparisons
4.1.2. Comparison Between Rest and Agonist-Antagonist Co-contraction
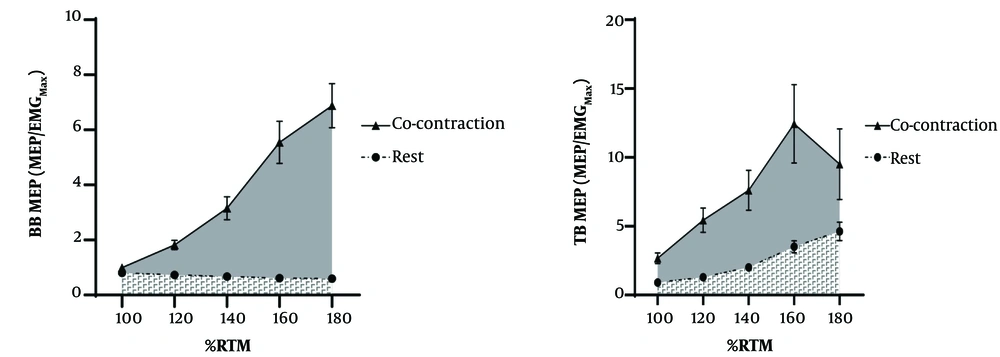
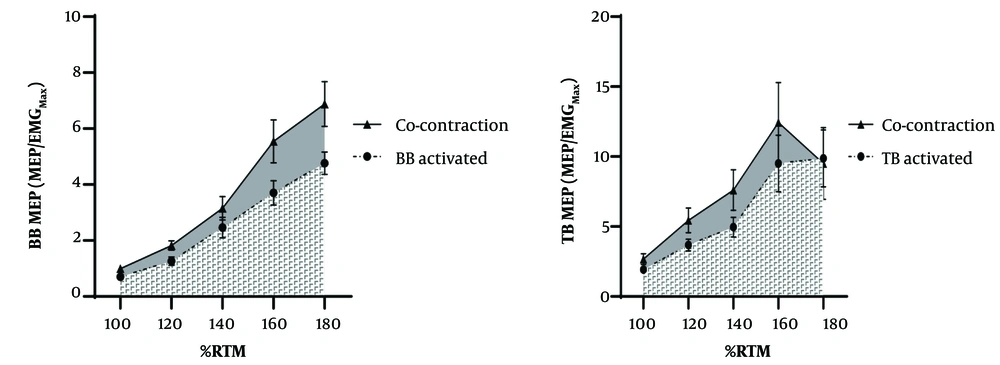
Pre-stimulus EMG RMS for the BB was significantly lower (Cohen's d: -1.10, P < 0.001) during rest (0.0024 ± 0.0005 mV) compared to MCo (0.022 ± 0.0026 mV). Similarly, the background EMG RMS for the TB was significantly lower (d: -1.15, P < 0.001) during rest (0.0023 ± 0.0006 mV) compared to MCo (0.0091 ± 0.0006 mV). Total AURC for the BB MEP was significantly larger (d: -4.15, P < 0.001) during MCo (87.59 ± 4.29 Au) compared to rest (40.17 ± 2.05 Au). Also, a large effect was observed for TB AURC (Cohen's d: -2.47, P < 0.001) during MCo (140.91 ± 13.65 Au) compared to rest (64.44 ± 5.03 Au) (Figure 3).
4.1.3. Comparison Between Rest and Antagonist Activation
Pre-stimulus EMG RMS for the BB was significantly lower (Cohen's d: -1.37, P < 0.001) during rest (0.0024 ± 0.0005 mV) compared to Antagonist Activation (0.022 ± 0.0026 mV). Similarly, the background EMG RMS for the TB was significantly lower (d: -1.53, P < 0.001) during rest (0.0023 ± 0.0006 mV) compared to Antagonist Activation (0.0091 ± 0.0006 mV). Total AURC for the BB MEP was significantly larger (d: -0.50, P = 0.015) during Antagonist Activation (45.91 ± 2.41 Au) compared to rest (40.17 ± 2.05 Au). Also, a large effect was observed for TB AURC (Cohen's d: -0.28, P = 0.377) during Antagonist Activation (73.26 ± 4.54 Au) compared to rest (64.44 ± 5.03 Au) (Figure 4).
4.1.4. Comparison Between Agonist Activation and Co-contraction
Pre-stimulus EMG RMS for the BB was not significantly different (Cohen's d: -0.71, P = 0.259) during Agonist Activation (0.022 ± 0.0026 mV) compared to MCo (0.022 ± 0.0026 mV). However, the background EMG RMS for the TB was significantly lower (d: -5.39, P < 0.001) during Agonist Activation (0.0091 ± 0.0006 mV) compared to MCo (0.0091 ± 0.0006 mV). Total AURC for the BB MEP was significantly larger (d: -2.39, P < 0.001) during MCo (87.59 ± 4.29 Au) compared to Agonist Activation (60.20 ± 2.96 Au). Also, a large effect was observed for TB AURC (d: -1.28, P < 0.001) during MCo (140.91 ± 13.65 Au) compared to Agonist Activation (101.00 ± 6.41 Au) (Figure 5).
5. Discussion
The results of the present study indicated that simultaneous activation of agonist-antagonist muscles (BB and TB) significantly increases the MEP in the arm muscles. For both the BB and TB, compared to conditions when both muscles were at rest, MCo of the agonist-antagonist led to a larger MEP area under the curve. Our findings indicate that during a voluntary MCo task, the specific descending drive from MCo and the modulation of reciprocal inhibition contribute to larger MEPs by enhancing the descending neural volleys induced by TMS. The increase in MEP with greater agonist muscle activity in both the upper and lower limbs has been previously demonstrated (1, 29). However, there is limited evidence on whether antagonist MCo and varying intensities of TMS affect MEP amplitudes. In this study, we directly compared the impact of antagonist muscle activity on the MEP amplitude of agonist muscles at different TMS intensities.
A novel aspect of our approach was the comparison between simultaneous agonist-antagonist activation, resting conditions, and agonist-only activation. Our study provides three key novel contributions: (1) Unlike previous work that typically examined single conditions (e.g., rest vs. MCo), we systematically compared three distinct states (rest, agonist-only, and agonist-antagonist MCo); (2) while prior studies often used a single TMS intensity (9), we demonstrated the consistency of MCo effects across multiple stimulation intensities; and (3) we extended these investigations to upper-limb muscles (BB/TB), whereas most existing evidence came from lower-limb studies (9).
The increased MEPs during BB/TB MCo likely reflect both (1) spinal mechanisms and (2) cortical mechanisms. Modulations in spinal circuit excitability, influenced by activity, have been studied in both tonic (30) and dynamic (31) tasks, as well as during walking (2). However, only a few studies have used TMS to examine corticospinal excitability in agonist and antagonist muscles during voluntary muscle MCo tasks. At the spinal level, MCo (or MCa) is controlled by various mechanisms such as reciprocal inhibition (32) and presynaptic inhibition of Ia afferents (33). At the cortical level, modulation of the descending cortical drive within spinal inhibitory Ia interneurons plays a key role in enabling the simultaneous activation of antagonist muscles (30, 34). While certain corticospinal neurons may be specifically tuned to MCo activity (35), the increase in the AURC of MEPs indicates greater excitability in circuits related to MCo.
Although our study does not specifically identify the neural mechanisms controlling MCo in arm muscles, it does provide new evidence of heightened corticospinal excitability during agonist-antagonist MCo at all TMS intensities tested. Future TMS studies, combined with spinal reflex measurements or cervicomedullary stimulation and additional TMS parameters (paired-pulse such as SICI, LICI, and ICF), may help to further clarify the neural mechanisms underlying MCo. Additional evidence suggests that cortical activation during MCa differs from that seen during activation of the agonist muscle alone. Imaging studies have revealed a larger and more distinct activation pattern in the M1 during MCa compared to when either the agonist or antagonist muscle is activated individually (36). While this heightened activation could indicate the engagement of MCo-specific cortical networks, it may also reflect the increased cortical demand required by the complexity of the task. The relationship between MCa and cortical demand has been demonstrated (37) and it is influenced by the nature of the motor activity (36).
Our findings demonstrate that voluntary MCo of agonist-antagonist muscles robustly enhances corticospinal excitability across all TMS intensities, extending previous lower-limb results to upper-limb muscles. This effect has important implications for neurorehabilitation (e.g., enhancing motor recovery post-stroke), sports training (optimizing MCo exercises), and motor control research (understanding neural coordination mechanisms). Also, voluntary MCa of muscles like the BB and TB might be cognitively demanding enough to require greater cortical involvement, possibly engaging distinct descending pathways. Future research could explore how cognitive effort during MCo or skilled motor tasks affects TMS-induced MEPs. Unfortunately, our current data cannot discern whether the observed increase in MEP amplitude during MCo, compared to rest, results from differences in attentional focus or the complexity of the motor task itself.
5.1. Conclusions
This study provides valuable insights into the impact of antagonist muscle activation during TMS and offers essential methodological guidance for future TMS evaluations of upper limb muscles. We demonstrated that both antagonist activation and simultaneous agonist-antagonist activation lead to an increase in MEP amplitudes, with this effect observed consistently across all TMS intensities. These findings highlight the need for further investigation into MCo-related modulations of motor cortical excitability, particularly in different populations such as athletes or patients. Future TMS studies, combined with spinal reflex measurements and additional TMS parameters (paired-pulse such as SICI, LICI, and ICF), may help to further clarify the neural mechanisms underlying MCo.