1. Background
The demand for new antimicrobial agents is increasing due to the rise in multidrug-resistant bacteria. Consequently, researchers are exploring new therapeutic alternatives against these resistant strains (1). Plant derivatives have emerged as a potential new source, with these compounds acting in various ways to inactivate or block the growth of pathogens (2).
Momordica charantia, from the Cucurbitaceae family, features bright yellow flowers, and its fruit turns yellow or orange when ripe. It is rich in vitamin C (3). This species is notable for its antimicrobial (4), nutritional, and anti-inflammatory properties, and is used in developing countries for its healing properties for stomach ulcers (5), rheumatism, and more. M. charantia is employed in folk medicine to treat toothache, diarrhea, boils, cancer, high blood pressure, obesity, bacterial and viral infections, diabetes, and pneumonia (6).
Nerium oleander is an evergreen shrub belonging to the Apocynaceae family, widely distributed worldwide. It possesses antibacterial (7), antifungal (8), antidiabetic (9), antioxidant (10), and antitumor properties (10, 11).
Hyssopus officinalis is a member of the Lamiaceae family and is widely grown in Iran. The active ingredients of this plant help control blood pressure, aid digestion, and reduce stomach bloating. The most important compounds in its essential oil include pinocamphene, alpha- and beta-pinene, camphene, and sesquiterpene alcohols, tannins, bitter substances, diosmin, hyssop, and mucilage compounds. Its essential oil is bitter, pungent, dry, and slightly warming (12). This plant is used for various purposes, such as anti-inflammatory, antibacterial, antipyretic, antispasmodic, antihypertensive, and antilipidemic applications (13). Hyssopus officinalis has been shown to inhibit the growth of bacteria (14).
2. Objectives
The aim of this study was to investigate the antimicrobial properties of ethanolic extracts of M. charantia, N. oleander, Solanum nigrum, and H. officinalis on food pathogens.
3. Methods
The leaves of M. charantia, N. oleander, S. nigrum, and H. officinalis plants were collected from the plains of Sistan and Baluchestan province, Zabol city. The plant samples were dried in the shade and ground. Five grams of the ground powder was dissolved in 50 cc of ethanol and placed on a shaker for 24 hours. The extract was then filtered and concentrated using a rotary evaporator. The concentrated extracts were stored in the rotary evaporator until use.
3.1. Bacterial Strains and Culture Condition
The bacterial strains Staphylococcus aureus ATCC1189, Shigella dysenteriae ATCC1188, Listeria monocytogenes ATCC1298, Vibrio cholerae ATCC1611, and Bacillus cereus ATCC1015 were obtained from the Microbiology Laboratory of Zabol National University. The bacteria were cultured in a nutrient broth medium, and the microdilution method was used to examine the effect of the extracts on the bacteria. In the wells of a 96-well microplate, 10 microliters of nutrient broth medium were added. In the next step, 100 microliters of the desired plant extract, at different concentrations, were added, followed by 10 microliters of bacteria prepared at half McFarland dilution. The microplates were incubated for 24 hours, and the first clear well was considered as the minimum inhibitory concentration (MIC).
4. Results
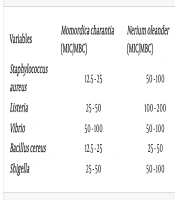
The lowest inhibitory concentration of the ethanolic extract of M. charantia was 12.5 mg/mL, which inhibited S. aureus and B. cereus strains at this concentration. The highest inhibitory concentration was 50 mg/mL, which inhibited Vibrio bacteria at this concentration. The results of the minimum inhibitory concentration of N. oleander showed that the lowest inhibitory concentration against B. cereus bacteria was 25 mg/mL (Table 1). The antimicrobial properties of H.officinalis revealed that the highest inhibitory concentration was 100 mg/mL, inhibiting Listeria and Shigella bacteria at this concentration, while the highest lethal concentration was 200 mg/mL (Table 1).
| Variables | MIC/MBC | |||
|---|---|---|---|---|
| Momordica charantia | Nerium oleander | Solanum nigrum | Hyssopus officinalis | |
| Staphylococcus aureus | 12.5 - 25 | 50 - 100 | 25 - 50 | 25 - 50 |
| Listeria | 25 - 50 | 100 - 200 | 12.5 - 25 | 100 - 200 |
| Vibrio | 50 - 100 | 50 - 100 | 50 - 100 | 50 - 100 |
| Bacillus cereus | 12.5 - 25 | 25 - 50 | 12.5 - 25 | 25 - 50 |
| Shigella | 25 - 50 | 50 - 100 | 50 - 100 | 100 - 200 |
Minimum Inhibitory Concentration and Minimum Lethal Concentration of Medicinal Plant Extracts Against Foodborne Pathogens
5. Discussion
Although the antibacterial properties of effective plant compounds, including essential oils and extracts, have been studied in the past, their mechanism of action in reducing or eliminating microbial load requires further investigation. While many chemical compounds in plants are similar, they do not have a specific mechanism for their effect on microorganisms; rather, each compound targets a specific site in the cell. The main factor in the antibacterial effect of plant extracts and essential oils is the chemical compounds that constitute them.
Sabzali investigated the antibacterial properties of the hydroalcoholic extract of N.oleander on pathogenic bacteria. The results showed that the most effective hydroalcoholic extract of oleander was at a concentration of 76 mg/mL. The largest diameter of the growth inhibition zone at this concentration was related to Enterococcus faecalis bacteria, and the smallest diameter was related to Pseudomonas aeruginosa. The results indicated that the lowest MIC was for S. aureus at a concentration of 5 mg/mL, and the highest MIC was for Escherichia coli and P. aeruginosa at a concentration of 76 mg/mL (15).
In the Hamoonnavard study, the antimicrobial effect of N. oleander on S. aureus and Staphylococcus epidermidis showed sensitivity to 40 and 80 μL of 25 mg/mL concentration, all concentrations of 50 mg/mL leaf extract, and all concentrations of 50 mg/mL flower extract (16). Additionally, the antibacterial properties of three ethanolic and aqueous petroleum extracts of Nerium oleander on four bacteria (B. subtilis, S. aureus, M. luteus, and P. aeruginosa) showed that the largest diameter of the growth inhibition zone was observed at a concentration of 100 mg/mL. The ethanolic extract had a greater effect on S. aureus and M. luteus with growth inhibition zone diameters of 18 and 14 mm, respectively. Pseudomonas aeruginosa and Bacillus subtilis were more sensitive to the aqueous extract, with inhibition zone diameters of 15 and 17 mm, respectively, at a concentration of 100 mg/mL (17).
In a study by Mouhcine, which investigated the antimicrobial activity of aqueous and ethanolic extracts of N. oleander, the results showed that the diameter of the inhibitory zone of the aqueous extract against Enterococcus faecalis was 10.0 ± 1.2 mm and against L. monocytogenes was 4.0 ± 1.0 mm, while the diameter of the inhibitory zone of the ethanolic extract against Enterococcus faecalis was 5.3 ± 0.6 mm (18). Another study showed that a concentration of 200 μg/mL of N. oleander essential oil inhibited the formation of P. aeruginosa biofilm (19).
Rajendra et al. found that the benzene extract had a higher inhibitory diameter (14 mm) than the ethanolic extract of N. oleander (11 mm) against B. subtilis (20). Saeidian et al. reported that the minimum inhibitory concentration and minimum bactericidal concentration of E. coli showed the highest sensitivity to the alcoholic extract of the leaves, with averages of 62.5 mg/mL and 125 mg/mL, respectively (21). Valizadeh et al. studied the anti-biofilm effect of ethanolic and acetone extracts of Karla extract, showing that the lowest inhibitory and lethal concentrations were 1.25 mg/mL and 2.5 mg/mL, respectively (22). Masithoh et al. found that the diameter of the inhibitory zone of Karla extract against Aeromonas hydrophila was 12.3 mm (23).
In a study of the effect of methanolic extract of S. nigrum leaves conducted by Zhao et al. on E. coli, S. aureus, B.subtilis, and Pasteurella multocida, they showed that this extract has a relatively moderate effect on these microbes (24).
5.1. Conclusions
The results of this study showed that the medicinal plant Karla is a better inhibitor than other plants for eliminating foodborne pathogens.