1. Background
Myocardial infarction (MI) is the most severe clinical manifestation of coronary artery disease, often requiring intervention through percutaneous coronary intervention (PCI) and antiplatelet medications (1, 2). An inflammatory response is known to initiate immediately after acute MI (3). Inflammatory cells such as neutrophils, monocytes, and macrophages rapidly infiltrate the injured myocardium, secreting proinflammatory cytokines, which may lead to additional myocardial damage (4). These inflammatory processes are recognized as key contributors to reperfusion injury, particularly in cases of ST-segment elevation myocardial infarction (STEMI) (5, 6). Consequently, it is crucial to explore therapeutic strategies aimed at modulating cytokine release in patients suffering from STEMI.
Colchicine is an alkaloid derivative from the autumn crocus with anti-inflammatory properties, traditionally used to treat several inflammatory diseases, including gout and familial Mediterranean fever (7). Its anti-inflammatory properties have led researchers to investigate its role in preventing complications associated with MI. There is a growing body of research on the therapeutic effects of colchicine on MI, with in vitro and in vivo studies demonstrating its cardioprotective effects (7). Although colchicine has recently been shown to have positive outcomes in patients with acute MI, reducing inflammation and infarct size (8, 9), the full spectrum of its clinical effects remains unclear, highlighting the need for additional randomized studies to address these unanswered questions.
2. Objectives
Moreover, while multiple studies have evaluated inflammatory factors among MI patients, no published studies have been conducted among the Iranian population. Therefore, we conducted a double-blind randomized controlled clinical trial to examine the hypothesis that administration of colchicine immediately after acute MI could lower levels of C-reactive protein (CRP), interleukin-1β (IL-1β), and IL-6 as key inflammatory cytokines, along with alleviating clinical manifestations such as systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate (HR).
3. Methods
3.1. Patients and Setting
This prospective, randomized, double-blinded study was conducted at the Department of Cardiology (Angiography Unit) of Hazrat-e Rasool General Hospital over one year, from May 2022 to May 2023. The study subjects included all referred adult MI patients (aged 18 to 80 years) with first-time STEMI, undergoing primary PCI, and presenting with chest pain onset for at least 12 hours. Patients were excluded if they were pregnant, breastfeeding, experiencing cardiogenic shock or hemodynamic instability, had preexisting active inflammatory or infectious diseases, severe liver failure (alanine aminotransferase > 3 times the upper limit of normal), renal failure (estimated glomerular filtration rate < 45 mL/min per 1.73 m2), history of using CYP3A4 inhibitors, thrombocytopenia or leukopenia, or any hypersensitivity reaction to colchicine. Additionally, patients with a history of myopathy, atrial fibrillation (AF), previous MI, coronary artery bypass graft (CABG), or MI in the context of stent thrombosis were excluded.
Baseline data, including age, gender, history of smoking, underlying diseases such as hypertension (HTN), diabetes mellitus (DM), hyperlipoproteinemia (HLP), family history of cardiovascular diseases (CVD), medications (including aspirin, beta-blockers, ACE inhibitors, and statins), complete blood count (CBC) including white blood cell (WBC) and neutrophil counts, and troponin I levels, were recorded at the beginning of the study and 24 hours after intervention in both groups.
3.2. Patient Randomization, Blinding, and Grouping
Patients were selected based on defined inclusion and exclusion criteria and assigned to two groups using random allocation software version 2.0 (1:1). All included patients were confidentially allocated to a group within the study. According to an arbitrary approach, the sample size was selected as 10% of patients with acute MI referred to the hospital. This study was double-blinded, with both patients and physicians unaware of the group assignments. Patients in the intervention group received 3 mg of colchicine (Modava Pharmaceutical Company, Iran) at the start of primary PCI and one hour afterward, while the control group received a placebo at the same times. Placebo tablets were manufactured to be identical to the colchicine tablets in the laboratory of the School of Pharmacy and Pharmaceutical Sciences, Islamic Azad University of Medical Sciences.
3.3. Biochemical Measurement
The primary outcomes of this study were the levels of inflammatory cytokines, including CRP, IL-1β, and IL-6, measured 24 hours after the first dose of the intervention. Ten milliliters of venous blood samples were collected from all included patients 24 hours after receiving the first dose of colchicine or placebo. Samples were centrifuged for 10 minutes, and plasma was isolated and frozen at -70°C until use. Sensitive enzyme-linked immunosorbent assay (ELISA) kits were used to determine plasma IL-1β and IL-6 levels (Demeditec Diagnostics GmbH, Germany). For evaluating IL-1β and IL-6, the minimum sensitivity detection was 0.35 pg/mL and 2 pg/mL, respectively, with inter- and intra-assay coefficients of variance of < 10% for each assay.
3.4. Statistical Analysis
Statistical analysis was performed using SPSS version 23 software. Qualitative variables were reported as number (percentage) and compared using the chi-square test. The normality of data distribution in quantitative variables was determined using the Shapiro-Wilk test and compared using either the Mann-Whitney test or Student’s t-test. In all cases, a P-value of less than 0.05 was considered statistically significant.
4. Results
4.1. Study Design
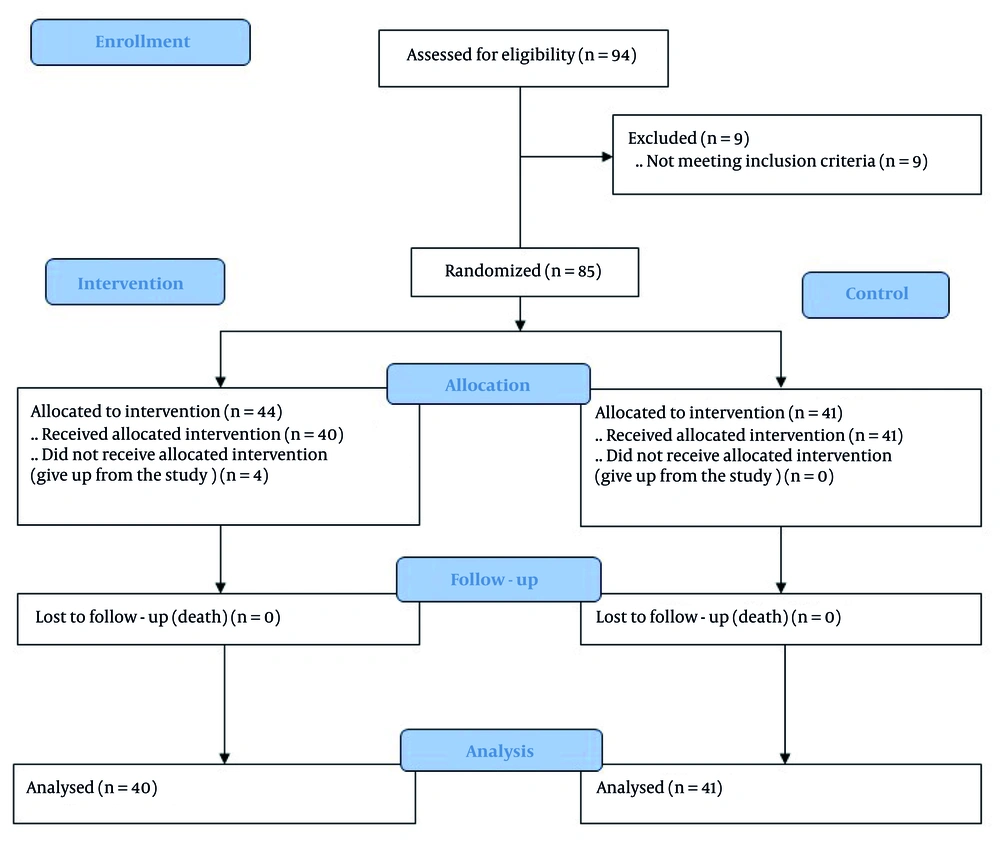
Ninety-four consecutive acute MI patients admitted to the angiography unit were evaluated for eligibility, and 85 patients met the study inclusion criteria. However, four patients were excluded due to administration errors. Ultimately, patients were randomized into the intervention (n = 40) and control (n = 41) groups, with no serious side effects detected in the study groups (n = 81) (Figure 1).
4.2. Demographic Information
Baseline patient characteristics, including demographics, underlying diseases, history of smoking, familial history of CVD, medications, and baseline laboratory data (admission troponin I, WBC count, neutrophil count, and CRP), were compared between the two groups. Our results showed that the mean age of patients was not statistically different between the two groups (55.00 ± 9.37 vs. 57.73 ± 10.60, P = 0.22). The gender ratio of the two groups was statistically similar (P = 0.71), and there was no significant difference in the history of smoking between the two groups (P = 0.65). Moreover, although the incidence of DM, HTN, HLP, and familial history of CVD differed between the two groups, the observed differences were not statistically significant (P = 1, P = 0.8, P = 0.1, and P = 1, respectively). Medications in both groups were also compared, revealing no statistical differences in the use of aspirin, beta-blockers, ACE inhibitors, and statins (P = 0.65, P = 1, P = 0.70, and P = 1, respectively). Baseline troponin, WBC, neutrophil, CRP, and IL-6 levels were statistically similar in both groups (P = 0.66, P = 0.70, P = 0.71, and P = 0.32, respectively). However, the IL-1β level in the intervention group was significantly higher compared to the control group at the beginning of the study (P = 0.001). On the other hand, although median values of SBP and HR were not different between the groups (P = 0.80 and P = 0.16, respectively), the baseline median DBP of patients in the intervention group was significantly higher than that of patients in the control group (P = 0.04) (Table 1).
| Characteristics | Total (n = 81) | Intervention (n = 40) | Control (n = 41) | P-Value |
|---|---|---|---|---|
| Age (y) | 56.38 ± 10.05 | 55.00 ± 9.37 | 57.73 ± 10.60 | 0.22 |
| Gender | 0.71 | |||
| Male | 74 (91.40) | 36 (90) | 38 (92.7) | |
| Female | 7 (8.6) | 4 (10) | 3 (7.3) | |
| Prior or current smoking | 0.65 | |||
| No | 35 (43.2) | 16 (40) | 19 (46.3) | |
| Yes | 46 (56.8) | 24 (60) | 22 (53.7) | |
| Underlying diseases | ||||
| DM | 15 (18.5) | 7 (17.5) | 8 (19.5) | 1.00 |
| HTN | 34 (42) | 16 (40.0) | 18 (43.9) | 0.8 |
| HLP | 10 (12.3) | 7 (17.5) | 3 (7.3) | 0.1 |
| Familial history of CVD | 1.00 | |||
| No | 42 (51.9) | 21 (52.5) | 21 (51.9) | |
| Yes | 39 (48.1) | 19 (47.5) | 20 (48.8) | |
| Drug history | ||||
| Aspirin | 44 (54.3) | 23 (57.5) | 21 (51.2) | 0.65 |
| Beta - blocker | 3 (3.7) | 1 (2.5) | 2 (4.9) | 1.00 |
| ACE inhibitor | 17 (21.0) | 9 (22.5) | 8 (19.5) | 0.79 |
| Statin | 43 (53.1) | 21 (52.5) | 22 (53.7) | 1.00 |
| Troponin I (ng/L) | 40000 (495 - 50000) | 40000 (495 - 50000) | 40000 (3085 - 50000) | 0.66 |
| WBC (× 109/L) | 8.09 ± 1.58 | 8.027 ± 1.59 | 8.162 ± 1.59 | 0.70 |
| Neutrophil (× 109/L) | 5.80 (4.1 - 7.6) | 5.80 (4.2 - 7.5) | 5.80 (4.1 - 7.6) | 0.80 |
| CRP (mg/L) | 79 (44 - 140) | 80.50 (48 - 134) | 78.00 (44 - 140) | 0.71 |
| Interleukin-1B (pg/mL) | 16.20 (1.6 - 68.0) | 18.10 (2.8 - 68.0) | 12.75 (1.6 - 30.1) | 0.001 |
| Interleukin-6 (pg/mL) | 11.90 (1.6 - 69.0) | 11.90 (1.6 - 48.0) | 11.80 (2 - 69.0) | 0.32 |
| PCI SBP (mmHg) | 148 (103 - 190) | 149 (103 - 190) | 148 (108 - 178) | 0.80 |
| PCI DBP (mmHg) | 90 (58 - 100) | 90 (65 - 100) | 90 (58 - 100) | 0.04 |
| PCI HR (BPM) | 90 (23 - 105) | 89 (23 - 104) | 92 (62 - 105) | 0.16 |
Baseline Characteristics of Myocardial Infarction Patients a
4.3. Clinical Outcomes
Twenty-four hours after the administration of colchicine, the CRP level in the intervention group decreased compared to the baseline level [80.50 (48 - 134) vs. 62.50 (39 - 115), P < 0.001], whereas the CRP levels in the control group remained statistically unchanged after the intervention [78.00 (44 - 140) vs. 78.00 (53 - 133), P = 0.188]. Moreover, there were significant differences between the CRP levels of patients in the intervention and control groups after the intervention (P = 0.001).
The median plasma IL-1β level was 18.10 (2.8 - 68.0) in patients in the intervention group, which was significantly higher compared to the IL-1β level in patients in the control group [12.75 (1.6 - 30.1), P = 0.001]. However, there were no significant differences in IL-1β levels in either the intervention or control patients after the intervention compared to baseline levels (P = 1). Additionally, there were no significant differences between the IL-6 levels of the intervention and control groups [11.90 (1.6 - 48.0) vs. 11.80 (2.0 - 69.0), P = 0.321].
Systolic blood pressure was also evaluated after the intervention, revealing that SBP in both groups was statistically similar [130 (98 - 145) vs. 130 (98 - 145), P = 0.99]. The observed decreases in SBP in both the intervention and control groups were statistically significant compared to baseline values (P < 0.001). Diastolic blood pressure was assessed, and there were no remarkable changes after the intervention between the study groups [80 (60 - 90) vs. 75 (62 - 87), P = 0.06]. The DBP in both the intervention and control groups significantly decreased after the intervention (P < 0.001).
Finally, HR was compared inter- and intragroup, showing that HR significantly decreased in both the intervention and control groups after the intervention (P < 0.001). The HR of patients in the intervention group was lower than that in the control group 24 hours after the intervention, but the observed decrease was not statistically significant [72 (62 - 104) vs. 74 (60 - 89), P = 0.07] (Table 2).
| Clinical Outcomes | Intervention (n = 40) | P-Value a | Control (n = 41) | P-Value a | P-Value b |
|---|---|---|---|---|---|
| CRP (mg/L) | < 0.001 | 0.188 | 0.001 | ||
| Before | 80.50 (48 - 134) | 78.00 (44 - 140) | |||
| After | 62.50 (39 - 115) | 78.00 (53 - 133) | |||
| Interleukin-1β (pg/mL) | 1 | 1 | 0.001 | ||
| Before | 18.20 (2.5 - 69.0) | 13.25 (1.8 - 31) | |||
| After | 18.10 (2.8 - 68.0) | 12.75 (1.6 - 30.1) | |||
| Interleukin-6 (pg/mL) | - | - | 0.321 | ||
| After | 11.90 (1.6 - 48.0) | 11.80 (2. - 69.0) | |||
| PCI SBP (mmHg) | < 0.001 | < 0.001 | 0.99 | ||
| Before | 149 (103 - 190) | 148 (108 - 178) | |||
| After | 130 (9 - 145) | 130 (98 - 145) | |||
| PCI DBP (mmHg) | < 0.001 | < 0.001 | 0.06 | ||
| Before | 90 (65 - 100) | 90 (58 - 100) | |||
| After | 80 (60 - 90) | 75 (62 - 87) | |||
| PCI HR (BPM) | < 0.001 | < 0.001 | 0.07 | ||
| Before | 89 (23 - 104) | 92 (62 - 105) | |||
| After | 72 (62 - 104) | 74 (60 - 89) |
Comparison of Clinical Outcomes After Intervention
5. Discussion
Our results indicated that colchicine therapy decreased CRP levels compared to baseline values, and CRP levels in the intervention group were markedly lower than those in the control group at the end of the study. Plasma levels of IL-1β were significantly higher in patients receiving colchicine than in those in the control group. The SBP, DBP, and HR of all patients after the intervention were significantly decreased compared to baseline.
Previous studies have elucidated that the pathology of various disorders, such as cardiovascular diseases (CVDs), results from inflammation, and the related mechanisms should be explored (10). It has been found that colchicine can inactivate the NLRP3 inflammasome to block IL-1β and IL-18 release in macrophages (11). Moreover, colchicine downregulates markers indicating vascular damage, such as plasminogen activator inhibitor-1 (PAI-1), soluble intercellular adhesion molecule-1 (sICAM-1), fetuin-A, and high-sensitivity C-reactive protein (hs-CRP) (12).
Hemkens et al., in a meta-analysis, suggested that results from previous studies on colchicine therapy for CVD are contradictory, and conducting clinical trials to investigate the effects of colchicine on heart diseases like MI is necessary (13). Deftereos et al. indicated that colchicine administration for 5 days lowered inflammatory markers (maximal neutrophil count and maximal CRP levels) in 151 STEMI patients 12 hours after angina treated with PCI (14). Another study by Shah et al. demonstrated that periprocedural colchicine (1.2 + 0.6 mg) decreased hs-CRP and IL-6 levels after PCI in 198 acute coronary syndrome (ACS) patients, but it did not reduce CVDs or myocardial injury after a 30-day follow-up (15).
Nidorf and Thompson indicated that low-dose colchicine (0.5 mg twice a day), independent of aspirin and atorvastatin, could decrease hs-CRP levels in patients with stable coronary artery disease (16). Vaidya et al. showed that colchicine (0.5 mg/day) combined with optimal medical therapy (OMT) for one year significantly decreased hs-CRP levels in 40 ACS patients (17). However, in another study by Akodad et al., it was declared that colchicine (1 mg/day) combined with OMT for one month could not effectively reduce levels of CRP, procalcitonin, and leukocyte count in 44 acute MI patients (18).
Hennessy et al. found that although 30-day administration of colchicine (0.5 mg/day) in 224 patients with acute MI was safe, it resulted in no changes in CRP and IL-6 levels and WBC count (8). Tardif et al. indicated that colchicine (0.5 mg/day) treatment markedly lowered the risk of ischemic CVD events in MI patients, but hs-CRP levels and WBC count after 6 and 12 months, respectively, remained unchanged between intervention and control groups (19). Alam et al., in a meta-analysis, indicated that colchicine treatment of patients with ACS and chronic coronary syndromes (CCS) results in lowered hs-CRP and clinical events (20). Consistently, another meta-analysis by Pan et al. showed that colchicine therapy decreases hs-CRP and IL-6 levels in CAD patients (21). Results from the COLCOT and LoDoCo-MI studies by Sun et al. demonstrated that low-dose colchicine administration could lower inflammation and the risk of recurrent CVDs after MI, and hs-CRP assessment could predict the effects of colchicine (22).
Robertson et al. showed that 24 hours after the administration of both colchicine (1 + 0.5 mg) and placebo, intracellular IL-1β levels decreased compared to baseline in 21 ACS patients (23). Martinez et al. conducted a randomized clinical trial among ACS patients and indicated that colchicine (1 + 0.5 mg) administered 6 to 24 hours before angiography significantly decreased transcoronary IL-1β, IL-6, and IL-18 levels (24). Moreover, Nidorf et al. found that the administration of low-dose colchicine (0.5 mg/day) combined with statin therapy and OMT successfully prevented CVD incidence among patients with stable coronary disease (25). Wu et al., in an in vivo study, showed that colchicine inhibits AF by blocking IL-1β-induced IL-6 secretion in a rat sterile pericarditis model (26). In a meta-analysis by Nogic et al., a 2-year follow-up of ACS patients showed that colchicine combined with guideline therapies reduces urgent revascularization, CVD events, and cerebrovascular accidents (27). However, in another study by Diaz-Arocutipa et al., it was shown that although colchicine was not able to decrease mortality, recurrent MI, or other CVD problems, it had no adverse drug effects in post-acute MI patients (28).
This study had some limitations that should be considered in future investigations. Our study had a low sample size, and it is suggested to include patients from different hospitals in a cohort population. Moreover, other confounding factors, such as medications after PCI, should be considered to validate the obtained results. Although this study evaluated two inflammatory factors 24 hours after colchicine administration, the evolution of the inflammatory panel with a longer follow-up period is suggested for future studies to confirm the obtained results.
5.1. Conclusions
It can be concluded that the administration of colchicine in two doses was able to decrease inflammatory responses after PCI in patients with acute MI. However, it is necessary to follow up with more patients over a longer period and consider other effective confounding factors.