1. Background
Glaucoma is the primary cause of permanent blindness globally (1). It is categorized into open-angle and closed-angle types. In the United States, over 80% of patients are diagnosed with open-angle glaucoma, while closed-angle glaucoma remains a significant cause of severe vision impairment. Globally, it is estimated that 68.56 million individuals have primary open-angle glaucoma (POAG), while 17 million have primary angle-closure glaucoma (2). Both types of glaucoma can be acquired as primary or secondary diseases.
In 2010, there were 60.5 million individuals diagnosed with glaucoma (2). By 2020, this number had risen to 79.6 million. Glaucoma is more frequently observed among African and Hispanic populations (3, 4). Patients with glaucoma may not display symptoms for several years, indicating that the actual prevalence of the disease is probably greater than the reported statistics.
This disease is often asymptomatic until the advanced stages. It causes damage to the optic nerve and manifests as a loss of the visual field (5). Vertical cup-to-disc ratio (VCDR) and elevated intraocular pressure (IOP) are two primary endophenotypes of POAG (6).
Prior genome-wide association studies (GWASs) have uncovered 127 loci associated with the condition, collectively elucidating 9.4% of the familial risk (7). Nonetheless, these loci capture only a small portion of the heritability, with numerous undiscovered high-risk loci and largely unexplored biological functions.
The three main genes associated with POAG include MYOC [HGNC:7610], OPTN [HGNC:17142], or TBK1 [HGNC:11584] (8, 9). The Glu50Lys missense mutation in the OPTN gene and DNA copy number variations (CNVs) in TBK1 are responsible for triggering early-onset familial normal-tension glaucoma (NTG) with an autosomal dominant pattern of inheritance (10, 11). The OPTN protein p.(Glu50Lys) enhances TBK1-OPTN binding and increases protein insolubility. This gene is associated with normal-pressure glaucoma and early adult-onset open-angle glaucoma (12).
Modern next-generation sequencing (NGS) technology enables the generation of millions of DNA sequences in a single reaction, overcoming the constraints of the Sanger method for gene-to-gene and exon-to-exon sequencing (13). In this way, NGS generates a large amount of data, and a large number of variants can be observed in each individual. Molecular genetic analysis can be used to diagnose this disease in its early stages, perform phenotypic-genotypic correlations, personalize treatment, provide prognosis, and offer genetic counseling (14).
2. Objectives
In this study, by examining the OPTN gene, we aim to identify possible mutations involved in glaucoma in two families from Khuzestan.
3. Methods
In this cross-sectional study, two families from Khuzestan province were studied. In the first family, the patient’s child was a 22-year-old boy who presented with symptoms of congenital glaucoma and corneal opacity. The second family under study consisted of relatives of the first patient (the uncle of the patient).
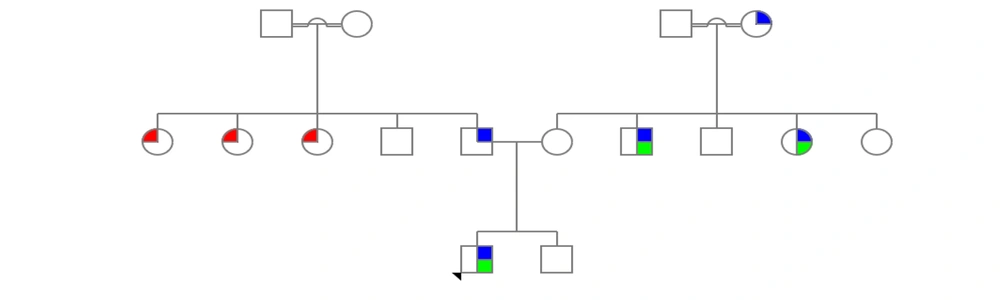
The second patient was also referred with the problem of a thin retina. This research was carried out in 2021 - 2022 at the Noorgen Medical Genetics Laboratory in Ahvaz. To enter the study, criteria such as the confirmation of clinical manifestations related to glaucoma by a specialist doctor and the consent of participants for research participation were required. After collecting clinical information and conducting genetic counseling, a family tree was drawn (Figure 1).
Genealogy of patient families related to OPTN gene. The examined family members include a father, a mother and a son who shows the patient's phenotype. The second family under investigation was the mother's family of the patient's child. In this family, the patient's uncle who showed similar symptoms was examined (the genealogy guide is shown on the right side of the image; green, corneal opacity; blue, congenital glaucoma; red, use of prescription glasses).
After collecting 5 mL of peripheral blood from the patient in a 0.5% EDTA tube, DNA extraction was performed by the salting-out method. To determine the quantitative and qualitative quality of DNA, a nano drop device and electrophoresis of samples on agarose gel were used, respectively. Genome sequencing was performed using the Illumina HiSeq 2500 platform. The resulting data were analyzed, and finally, the standard Sanger sequencing method was used to confirm the resulting variants with the genealogy pattern.
To perform PCR, 10 μL of Red Mix solution, 8 μL of double-distilled water, 2 μL of sample DNA, and 0.5 μL of reverse primers were poured into the reaction tube. The sequence of primers used in the research is given in Table 1. PCR products were electrophoresed (Figure 2).
| Name | Primer Sequence | Temperature (°C) | Product Length (bp) |
|---|---|---|---|
| OPTN ex8 F | 5. TTGGAGAATGTTCTGGAAAGC.3 | 59.3 | 485 |
| OPTN ex8 R | 5. CAGAAAGCACATTGCTTGGA.3 | 60.0 |
The Sequence of Reverse Primers Amplifying the Fragment Containing the Desired Mutations
The PCR products were read with an ABI 3130XL sequencing machine, and the results were analyzed using Chromas software and compared with reference sequences from the NCBI and ENSEMBL sites.
4. Results
In this research, two families from Khuzestan province were investigated. After analyzing the results and drawing the family tree, the type of mutation in the family was evaluated. In this family, a 22-year-old male with phenotypic symptoms of congenital glaucoma and corneal opacity was examined, which was confirmed by an ophthalmologist.
The second family consisted of the maternal relatives of the first patient. In this family, a patient with the same phenotype was referred. Pedigree examination showed that other family members and relatives exhibited similar symptoms, including retinal thinning and the use of prescription glasses. The results of WES identified 256,987 variants, and finally, after filtering the variants based on zygosity and the frequency of alleles in the population, one variant was selected as the molecular cause of the disease.
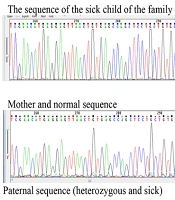
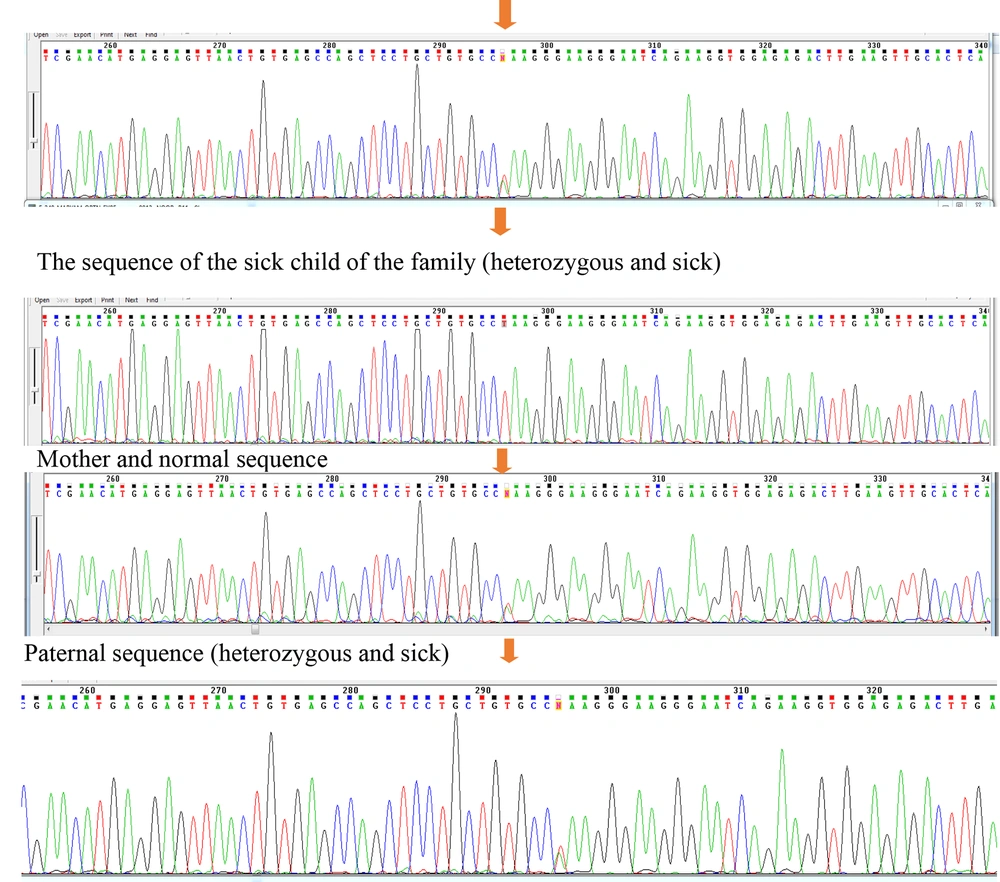
Examining the sequences of coding exons showed that this variant includes NM_001008213:exon8:c.T719A:p.L240Q, located in exon 8 of the OPTN gene. This variant can interfere with the function of the protein by altering the translation of the codon and ultimately changing the structure of the protein. Data from the ClinVar and HGMD bioinformatics databases declared the NM_001008213:exon8:c.T719A:p.L240Q variant as a VUS (variant of uncertain significance). However, bioinformatics studies have shown the variant to be possibly pathological (malignant). The results of checking the mentioned variants in the Online Mendelian Inheritance in Man (OMIM) database show that the mutations found can lead to glaucoma. The data obtained from the sequence show the substitution of base A with base T in the variant NM_001008213:exon8:c.T719A:p.L240Q in a heterozygous form in patients (Figure 3).
The sequencing results of parents with heterozygous genotype for the father, normal for the mother, one of the affected children with the heterozygous genotype and the uncle of the patient with the heterozygous genotype (patient). The sequence results in all samples are similar in the area and codon number and the only factor causing heterozygosity or homozygosity of individuals is the difference in the type of nucleotide in codon number 719.
5. Discussion
Glaucoma is an ocular condition marked by the gradual deterioration of the optic nerve, ultimately resulting in permanent loss of vision (15). The primary type of this condition is POAG, which is linked to elevated pressure inside the eye. A variant of POAG, known as NTG, is characterized by progressive damage to the optic nerve despite normal IOP. Mutations in the OPTN gene, responsible for producing optineurin, were the first genetic anomalies associated with NTG. These mutations include changes such as H26D, E50K, E103D, T202R, A336G, A377T, and H486R5. The H26D mutation was initially detected in Japanese individuals with POAG (16).
Genetic research has revealed that POAG exhibits genetic diversity and arises from various susceptibility genes along with potential environmental influences (17).
In the 5'-untranslated region, the human OPTN gene consists of three non-coding exons and 13 exons that code for a protein comprising 577 amino acids. Several isoforms, generated through alternative splicing, have been identified in this region, yet they exhibit identical characteristics. Examination of the OPTN sequence reveals that the encoded protein possesses numerous coiled structures, including a leucine zipper from amino acids 143 to 164, as well as a terminal finger (18).
The OPTN is present in various tissues, including the heart, brain, liver, skeletal muscle, kidney, pancreas, and eye (19, 20). The results of the present study showed that the variant NM_001008213:exon8:c.T719A:p.L240Q, obtained from the exome analysis of the patient in the OPTN gene, corresponds to the clinical symptoms of glaucoma. The results of Sanger sequencing in the patient's parents confirm the presence of the pathogenic variant in the family.
The results of the examination of the second family, which is the maternal family of the first patient, also confirm the presence of the pathogenic variant in the family tree. Finally, the analysis of the obtained sequences and the genealogical pattern of the investigated families confirms the hypothesis of the pathogenicity of the obtained variant.
Goa et al., in his research, extensively discussed the relationship between the M98K optineurin type (OPTN) and POAG. The researchers conducted a comprehensive meta-analysis to explore the link between the M98K variant and POAG alongside its subcategories. They systematically reviewed articles from various databases such as PubMed, Embase, Web of Science, and the China National Knowledge Infrastructure (CNKI) to gather relevant studies on the association between the M98K variant and POAG, spanning from the earliest publications to December 31, 2019. For their study, a total of 34 qualifying articles comprising 7,310 individuals with POAG and 5,173 control subjects were included in the meta-analysis. The combined findings indicated a notable correlation between the M98K variant and the overall susceptibility to POAG based on the dominant model. Subgroup evaluations, however, did not reveal any significant connection between M98K and the risk of NTG, high-tension glaucoma (HTG), juvenile-onset open-angle glaucoma (JOAG), adult-onset POAG, Asian POAG, or non-Asian POAG. In conclusion, the updated meta-analysis highlighted that the OPTN M98K variant exhibited a significant association with susceptibility to overall POAG (21).
The results of this research are consistent with the present study and indicate the importance of the OPTN gene in glaucoma. The findings will help to complete the genetic panel affecting this disease by identifying the new variant with the help of the NGS technique. On the other hand, according to the data from databases such as OMIM and Mutation Taster, the identified mutation causes genetic vision disorders.
5.1. Conclusions
In conclusion, it can be stated that by using the NGS technique, unknown variants associated with glaucoma disease can be investigated, thereby contributing to the completion of the genetic diagnostic panel for this disease. In this research, the pathogenic variant NM_001008213:exon8:c.T719A:p.L240Q was identified, and bioinformatics studies, along with matching the genealogical pattern of the investigated families, also confirmed this finding.