1. Background
Polycystic ovary syndrome (PCOS) is the most common disorder among young women, increasing the risk of chronic diseases such as heart disease, diabetes, and reproductive problems (1). Initially considered a reproductive disorder, PCOS is now recognized as a metabolic and psychological disorder (2). The average prevalence of PCOS is 21.27%, and the proportion of women with PCOS has increased over the past decade (3). The diagnosis of PCOS is considered in women with amenorrhea or irregular menstrual cycles, hyperandrogenism, or infertility. The Rotterdam diagnostic criteria require two of the following factors: Clinical or laboratory indications of hyperandrogenism, oligo- or anovulation, and polycystic ovarian morphology on sonogram (4).
Androgen excess is a common feature of PCOS, and AR signaling pathways play a critical role in its pathogenesis (5). Androgens are synthesized in ovarian theca cells in response to luteinizing hormone (LH) and act as ligands to AR in the granulosa cells (GC) (6). Previous studies in humans and primates have shown that follicle-stimulating hormone receptor (FSHR) and AR gene expression in GC is strongly associated with follicular development. Furthermore, knockout of the AR in GC in mice affects fertility, arrests follicle development, and leads to premature ovarian failure (6). Androgens exert their effects through AR, a nuclear transcription factor that acts as a ligand-activated regulator by binding to androgen response elements (AREs) located in the promoter and enhancer regions of target genes (5, 7). This gene is situated on the X chromosome, specifically at the Xq11-12 region (8).
The AR protein consists of four functionally distinct domains encoded by eight exons: Exon 1 encodes the N-terminal transactivation domain; exons 2 and 3 encode the DNA-binding zinc finger domains; exon 4 encodes the hinge domain (HD); and exons 4 to 8 collectively encode the ligand-binding domain (9). Exon 1 of the AR gene harbors a highly polymorphic CAG trinucleotide repeat, which encodes a polyglutamine tract within the N-terminal transactivation domain (10). The number of CAG repeats may vary among different ethnic groups from 8 to 35, and a hemizygous expansion beyond the normal repeat range (≥ 35 CAG repeats) in males causes X-linked spinal and bulbar muscular atrophy (SBMA) (11).
According to in vivo research, there is an inverse relationship between the polyglutamine tract length in exon 1 of the AR and its functional performance. A longer polyglutamine tract corresponds to lower AR transactivation activity, while fewer repeats result in higher AR activity (12, 13). The transcriptional activity of the AR depends on interactions between the two termini (N/C-interaction) of this receptor and its co-activator; longer polyglutamine tracts have been shown to inhibit these interactions (6). While many studies have explored the link between CAG repeat length in the androgen receptor (AR) and PCOS, the results have been inconclusive — some studies reported a significant association (14-17), whereas others found no such relationship (18, 19).
2. Objectives
The present study aimed to investigate the correlation between the length of CAG repeats in the AR gene and the risk of PCOS in Iranian women.
3. Methods
Ethical approval for the study was granted by the Ethics Committee of Shahrekord University of Medical Sciences, Iran (91-01-74-1504). The trials were conducted between 2014 and 2015 at the Cellular and Molecular Research Center, Shahrekord University of Medical Sciences, Shahrekord, Iran. All subjects were recruited from health centers in Shahrekord, Iran, and informed consent forms were completed by all participants. The inclusion criteria for this study were based on the modified Rotterdam standard. Individuals diagnosed with congenital adrenal hyperplasia, Cushing’s syndrome, thyroid dysfunction, or hyperprolactinemia were excluded from the study.
Hyperandrogenism is defined as elevated levels of androgens, such as testosterone, in females. Hirsutism is diagnosed when excessive hair growth occurs in areas typically seen in men, such as the face, back, chest, and abdomen. Patients were asked about their menstrual cycle history, and the length of their cycles was investigated. Ovarian morphology was assessed by ultrasonography, with follicle excess being the main symptom of polycystic ovarian morphology. A total of 51 patients met the specified inclusion and exclusion criteria, while 58 healthy control subjects of similar age and ethnicity were recruited. The control group consisted of individuals with confirmed fertility, regular menstrual cycles, normal ovarian morphology, and no history of sub-fertility treatments (20). No significant relationship was found between the patient and control groups.
3.1. DNA Isolation
Peripheral blood samples (3 - 5 mL) were collected from all participants, and genomic DNA was extracted using the standard phenol-chloroform method. DNA quality was assessed using a NanoDrop Spectrophotometer (NanoDrop 2000, Thermo Scientific) by measuring optical density (OD) ratios at 260/280 and 260/230 nm.
3.2. PCR Amplification and Genetic Analysis
The CAG repeat region of the AR gene was amplified using the primers listed in the table. PCR cycling conditions included an initial denaturation at 95°C for 5 minutes, followed by 35 cycles of 95°C for 50 seconds, 61°C for 50 seconds, and 72°C for 40 seconds, with a final extension at 72°C for 8 minutes. The PCR products were purified, and allele sizing was performed using GeneMarker V2.6.7 (Demo).
3.3. Statistical Analysis
Data were analyzed using SPSS version 24.0 (SPSS Inc., Chicago, IL, USA). Initially, CAG repeat lengths were treated as continuous variables, followed by analysis as categorical variables. Student’s independent t-test was applied to compare the mean bi-allelic CAG repeat lengths between cases and controls for continuous data. For categorical data, the chi-square test was used to compare the frequency distribution of bi-allelic CAG repeats between the two groups. Two-sided P-values ≤ 0.05 were considered statistically significant, with a 95% confidence level. Results are presented as mean ± standard deviation (SD).
4. Results
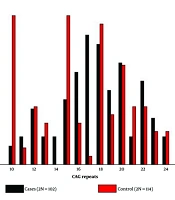
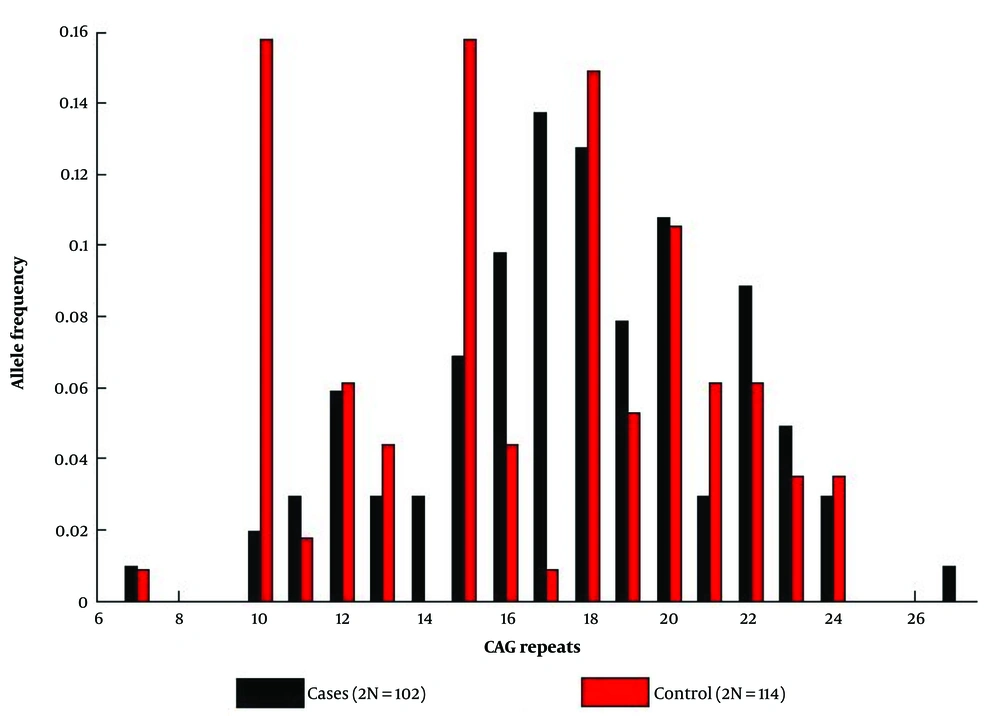
The pattern of allelic distribution of CAG repeat is shown by Figure 1. The number of CAG repeat in our population was between 7 and 27 for cases and between 7 and 24 for control subjects.
Table 1 presents the bi-allelic mean CAG repeat lengths for both controls and cases, which ranged from 10 to 23.5 in each group, with no significant difference found between them (P = 0.189).
| Groups | No. | Mean ± SD | P-Value a |
|---|---|---|---|
| Controls | 55 | 16.61 ± 4.14 | 0.18 |
| Cases | 51 | 17.58 ± 3.38 |
Comparison of CAG Bi-allelic Mean Length Between Controls and Case Subgroups
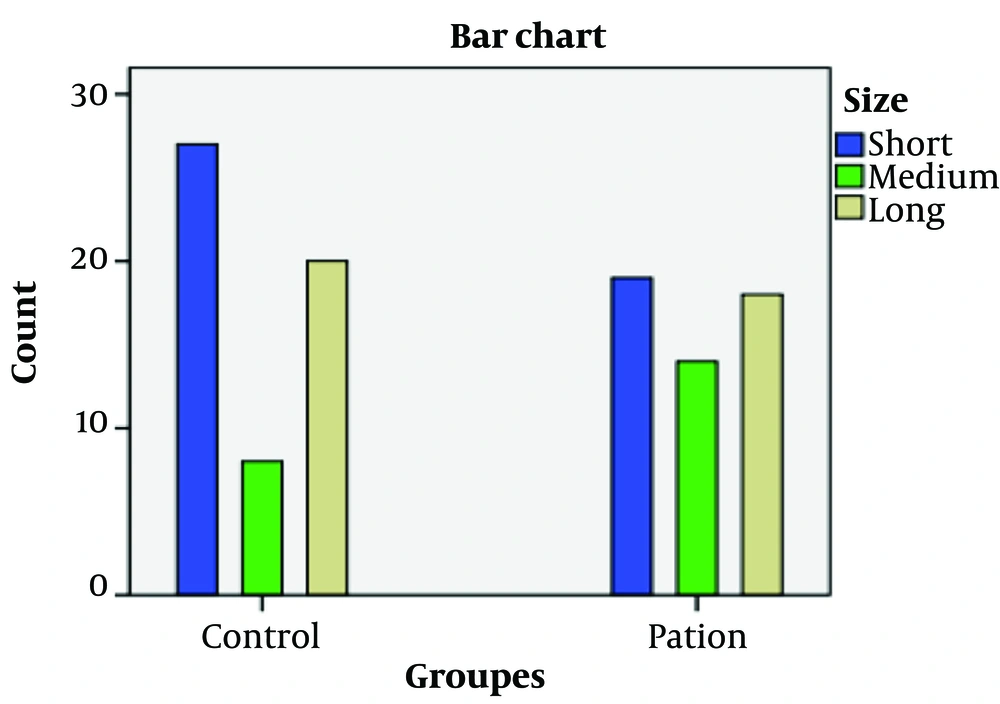
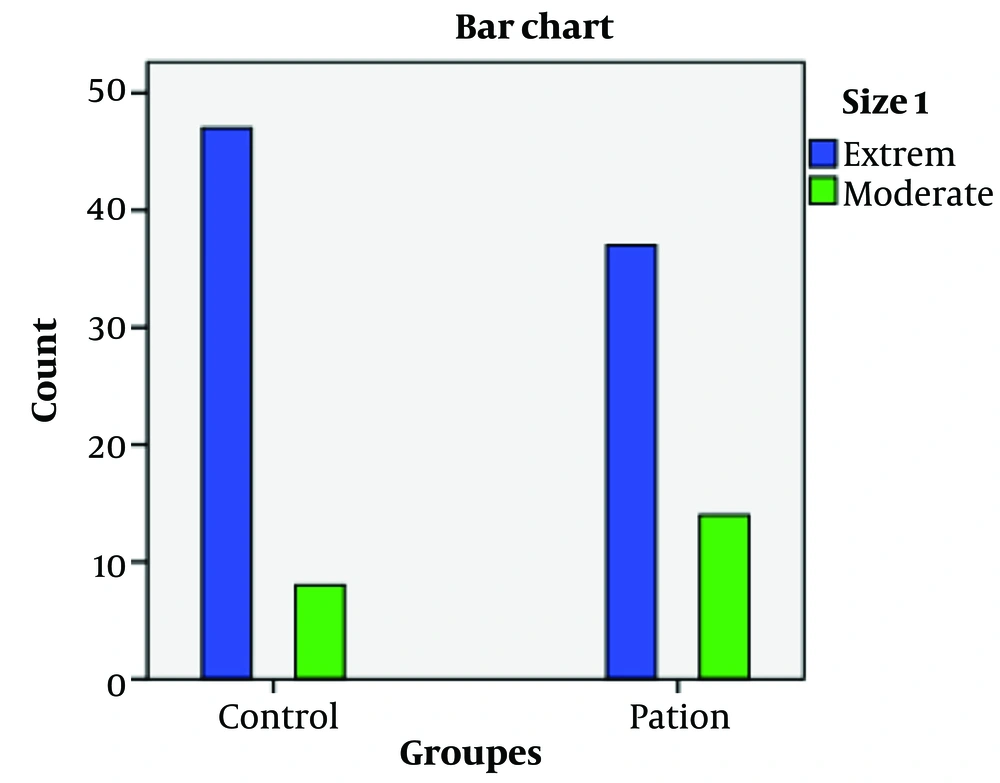
The frequency distributions of the bi-allelic mean CAG repeat lengths were categorized into three groups (short, moderate, and long) as shown in Table 2 and Figure 2, and into two groups (extreme and moderate) as presented in Table 3 and Figure 3, respectively. Comparison of the frequency distributions in categorical variables between groups revealed that the short and extreme alleles in controls were approximately 12% and 13% more frequent than in cases, respectively, while the moderate allele was 13% more frequent in cases than in controls. However, these frequencies did not show a statistically significant difference between groups.
| Groups | No. | Short CAG (< 17) | Moderate CAG (17 - 19) | Long CAG (> 19) | X2 Value | P-Value b |
|---|---|---|---|---|---|---|
| Controls | 55 | 27 (49.1) | 8 (14.5) | 20 (36.4) | 2.98 | 0.22 |
| Cases | 51 | 19 (37.3) | 14 (27.5) | 18 (33.3) |
Percentage Frequency Distribution of Bi-allelic CAG Mean Length Across Three Categories (Short, Moderate, and Long) a
| Groups | No. | Extreme CAG (< 17 and > 19) | Moderate CAG (17 - 19) | X2 Value | P-Value b |
|---|---|---|---|---|---|
| Controls | 55 | 47 (85.5) | 8 (14.5) | 2.68 | 0.1 |
| Cases | 51 | 37 (72.5) | 14 (27.5) |
Percentage Frequency Distribution of Bi-allelic CAG Mean Length in Two Categories: Extreme and Moderate a
5. Discussion
The PCOS is a multifactorial and heterogeneous condition. Despite numerous studies, the etiology of PCOS largely remains unknown. Previous research has demonstrated that androgen excess is an important factor in the etiology of PCOS. The variation in AR CAG repeat length is an intriguing subject, with many studies conducted to determine the correlation between this polymorphism and the risk of PCOS. The contradictory results of these studies in various populations prompted us to conduct this investigation for the first time in an Iranian population. Our results indicate no significant difference in CAG repeat length between the case and control groups.
Previous studies have employed various analytical approaches, including continuous data analysis — comparing the bi-allelic mean CAG repeat length between groups; dichotomous analysis — categorizing CAG repeat lengths into two groups (extreme and moderate) and examining allele and genotype distributions; and nominal analysis — dividing CAG repeat lengths into three categories (short, moderate, and long) to explore potential associations. In this study, we applied all of these methods to ensure a thorough and comprehensive analysis. In dichotomous data analysis, extreme and moderate alleles have lengths of < 17, > 19, and 17 - 19, respectively. In short, moderate, and long categories, alleles have lengths of < 17, 17 - 19, and > 19, respectively (21).
In exon 1 of the AR gene, in addition to the CAG repeat, there is another polymorphism, the GGC repeat, and the encoded polyglycine is continued at the N-terminus of the AR protein. Some previous studies demonstrated that AR function can be modulated by certain combinations of CAG and GGC triplets, whereas the length of CAG and/or GGC repeat numbers alone showed no correlation with AR activity. Ferlin et al. demonstrated certain haplotypes of CAG/GGC that have susceptibility or protective effects on infertility. The susceptibility alleles were CAG = 21/GGC = 18 and CAG ≥ 21/GGC ≥ 18, with relative risks of 2.47 and 1.6, while the protective alleles were CAG ≥ 23/GGC ≤ 16, with a relative risk of 0.09. In 2014, Deli Muti et al. (21) suggested a correlation between the allele CAG 22/GGC 17 and a defect in sperm production, suggesting that certain combinations of CAG and GGC triplets affect AR function; however, this needs to be verified in more studies (22, 23).
Several in vitro studies have reported conflicting findings regarding the relationship between CAG repeat polymorphism in the AR gene and AR activity levels. Some studies have shown an inverse association between AR CAG repeat length and AR activity, but other in vitro studies have shown no association (24). Numerous studies have explored the association between CAG repeat polymorphism in the AR gene and the risk of PCOS. Some researchers have reported a significantly higher frequency of short CAG alleles in PCOS patients compared to controls (25), while others have found no correlation between this polymorphism and PCOS susceptibility (26). These inconsistent results could be due to differences in ethnic/racial backgrounds and criteria for recruitment of case and control subjects.
As we know, to compensate for dosage, one of the two X chromosomes is randomly inactivated in all somatic cells of females; the locus of the AR gene is on the X chromosome. However, it would be better to evaluate X chromosome inactivation (XCI) to ensure that this process occurs normally in all subjects. As some studies have shown that certain haplotypes of CAG/GGC triplets affect AR function, we suggest that the correlation between certain combinations of CAG and GGC triplets and PCOS should be evaluated. Previous studies showed that in addition to the genotype of the AR gene and the epigenetic effect of XCI, the coactivators and corepressors of the AR gene determine the activity of this gene, so the interaction of these coregulators with the polymorphism of the CAG repeat should be identified.
5.1. Conclusions
Our findings suggest that there is no significant correlation between the length of CAG repeat polymorphism in the AR gene and the risk of developing PCOS in Iranian women. Although certain allele length distributions differed between cases and controls, these variations were not statistically strong enough to establish a definitive association. Further studies with diverse populations and larger sample sizes are recommended to clarify the potential role of AR gene polymorphisms in the pathogenesis of PCOS.