1. Background
Chronic non-communicable diseases, especially cardiovascular diseases (CVDs), are the main contributors to global mortality and morbidity (1). Approximately 23.3 million individuals may be impacted by these conditions by 2030 (2). Additionally, in Iran, the risk of CVDs has increased from 2000 to 2016. Without further modification of risk factors, this trend is expected to continue until 2030 as well (3). Presently, there is particular concern regarding the impact of hypertension as a significant risk factor for CVDs (4). Hypertension is a multifactorial trait whose pathophysiology is associated with increased age, diet, obesity, physical inactivity, insulin resistance, inflammation, and oxidative stress (5). Specifically, increased bioavailability of reactive oxygen species (ROS) (referred to as oxidative stress) due to an overproduction of ROS, diminished levels of nitric oxide (NO), and reduced antioxidant capacity in the renal, nervous, and cardiovascular systems, along with an increase in inflammatory processes, vascular or endothelial abnormalities (6), and irregularities of coagulation, have been implicated in the pathogenesis of hypertension (7).
Superoxide dismutase (SOD) is one of the important antioxidant enzymes that plays a crucial role in protecting vasculature from the damaging effects of ROS, particularly superoxide radicals (8). Additionally, this enzyme inhibits the oxidative inactivation of NO, which is a crucial vasodilator (9). On the other hand, fibrinogen, a 340 kDa glycoprotein, is one of the key proteins involved in blood coagulation, platelet aggregation, and increased blood viscosity (10). Among the effective factors in thrombogenesis and CVDs, including tissue-type plasminogen and plasminogen inhibitor-1, fibrinogen seems to be the most vulnerable target for oxidant attack (11) and has the most impact as a cardiovascular risk factor (10). Plasminogen activator inhibitor 1 (PAI-1) is another biomarker that is interesting to investigate in CVD (12). The PAI-1 belongs to the superfamily of serine protease inhibitors (serpins). It is also the main inhibitor of the plasminogen activators, urokinase-type plasminogen activator (uPA), and tissue plasminogen activator (tPA). The balance of the hemostatic system can be disrupted by turbulence at these levels, leading to bleeding or thrombotic complications. The PAI-1's role in CVD is well-documented, which is not surprising (13).
Another factor implicated in the regulation of hypertension is the blood pressure (BP)-lowering peptide apelin, which is an adipokine and a peptide hormone secreted by visceral fat tissue (14). The apelin-APJ pathway has been recognized as a potent regulator of BP and blood flow in the vasculature and heart (15). Additionally, apelin acts as both an arterial and venous dilator, and these effects appear to be mediated through NO signaling (16). According to an experimental study, in wild-type mice, apelin has been shown to protect against cardiac fibrosis and vascular remodeling by directly regulating the expression of the PAI-1 gene. This protective mechanism involves the synergistic inhibition of angiotensin II signaling and an increase in the production of NO by apelin (17). Furthermore, it has been proven that there is a robust and independent association between low plasma levels of albumin and CVDs (18). Serum albumin levels have protective properties, such as preventing apoptosis, maintaining physiological homeostasis, antioxidant activity, and anti-inflammatory effects (19). Research shows that the fibrinogen to albumin ratio (FAR) is elevated in hypertension and atherosclerosis, indicating systemic inflammation linked to the severity of coronary artery disease (20, 21). Recent studies suggest that FAR is more strongly associated with clinical outcomes than fibrinogen or albumin alone (22). Modification of the aforementioned risk factors is an effective method to reduce CVD risk, and most cardiovascular risk factors can be altered with lifestyle changes (including engaging in regular physical activity, quitting smoking, moderating alcohol intake, maintaining a healthy Body Mass Index (BMI), and adopting a healthy diet) and medications (23).
Nowadays, exercise training is widely recommended as a key non-pharmacologic therapy for hypertension, particularly in older adults who are not as physically active as those with normal BP (24). Aerobic training (AT) has demonstrated efficacy in the treatment and prevention of CVDs and hypertension (25). Additionally, resistance exercise training has been shown to have positive effects on strength, hypertrophy, muscle function, and oxidative stress regulation (26). On the other hand, stretching training (ST) interventions have been found to improve vascular endothelial function and reduce arterial stiffness (27), with notable effects on apelin (28) and PAI-1 levels (29). Combined training, including both resistance and aerobic components, has been reported to increase SOD activity, reducing oxidative stress and plasma fibrinogen levels (30). Therefore, a combination of the exercises mentioned above is expected to have a potential positive effect in mitigating anticoagulant and fibrinolytic factors and complications of hypertension. People, especially those with physical disabilities, face barriers to accessing gym-based training (GBT) (31). Developing home-based training (HBT) programs as an alternative can alleviate psychological, environmental, and socio-economic barriers while providing similar physiological benefits (32). Also, the effectiveness of GBT versus HBT in patients with primary hypertension is not yet clear.
2. Objectives
Therefore, the main purpose of this study was to investigate the effect of simultaneous exercise training (i.e., resistance, aerobic, and stretch exercise) in the gym and at home on the aforementioned biochemical variables (SOD enzyme activity, plasma levels of fibrinogen, apelin, PAI-1, albumin, and FAR ratio) in men with primary hypertension. We hypothesized that simultaneous exercise training conducted both in gym and home settings would positively influence the CVD risk factors in men with primary hypertension.
3. Methods
3.1. Ethical Approval, Participants, and Study Design
In the present study, we used banked plasma samples originally collected in 2018 as part of a previously approved and completed research project (approval number: IR.BUMS.REC.1397.283, approval date: 2018-12-29). Participants in the original study agreed that their plasma samples could be used for our future research projects. However, we obtained new ethical approval code from the Ethics Committee of the University of Birjand in two phases (approval numbers: IR.BIRJAND.REC.1401.001 and IR.BIRJAND.REC.1401.002, approval date: 2022-12-28) for a new analysis of the existing samples. Additionally, this research has been documented in the Iranian Registry of Clinical Trials database as a clinical trial study (IRCT20190317043080N1 on 16th June 2019, updated on 5th February 2023). We have already published two papers related to this protocol, focusing on other aspects of hypertension risk factors (33, 34).
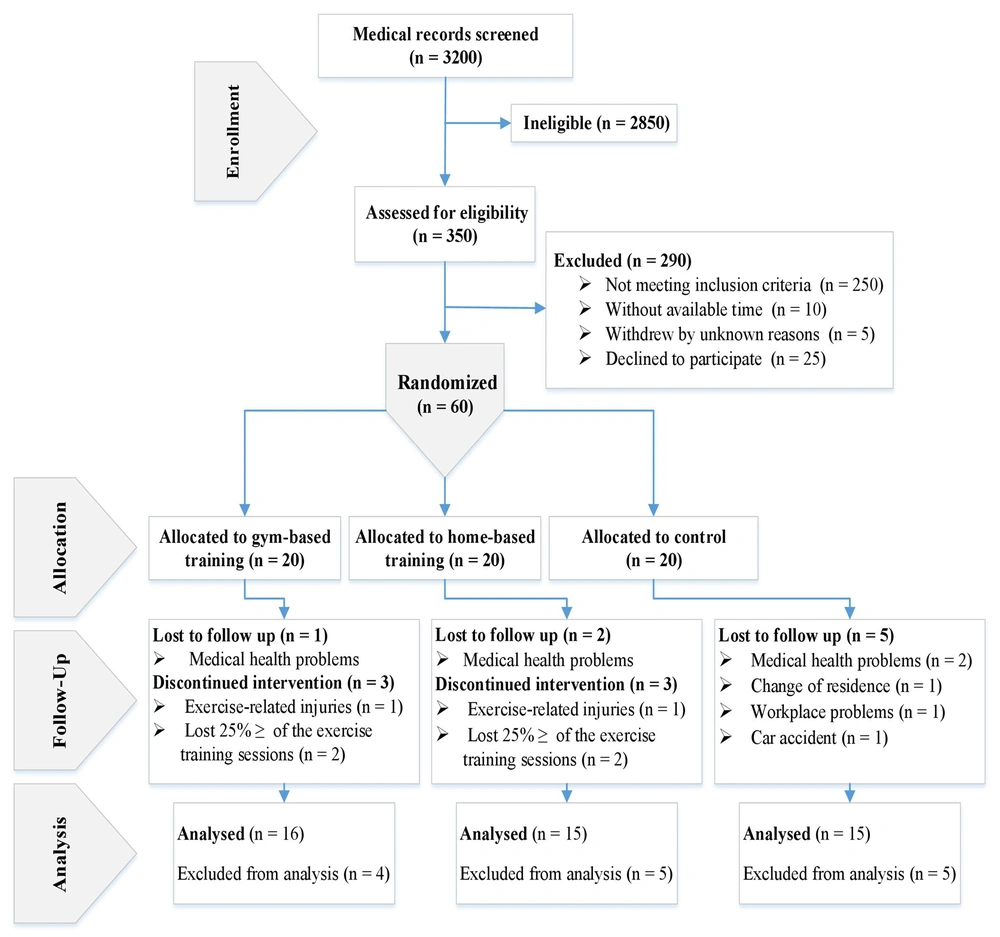
Before data collection began, every participant was briefed on the study’s methods, possible risks, and potential benefits. They then provided their formal written consent, adhering to the principles outlined in the Helsinki Declaration and approved by our Institutional Review Board. Considering the previous explanations, during the enrollment period from November 22 to December 21, 2018, we referred to the cardiovascular research unit at Dr. Toba Kazemi's cardiology clinic, situated within the Rezvan Medical Complex in Birjand, Iran. We reviewed the medical records of 3200 male patients who had received treatment between 2016 and 2018. Subsequently, during the recruitment period (December 21 to December 28, 2018), we invited 350 eligible selected patients (30 - 70 years old) to participate in the study via phone calls. Finally, 60 volunteers were selected according to the study entry criteria (Box 1), and 290 patients were excluded (Figure 1).
| Inclusion Criteria |
| - Based on physician diagnosis, at least one year suffering from primary hypertension |
| - Having systolic BP at 130 - 159 mmHg and diastolic BP at 80 - 99 mmHg (35) |
| - Do not take more than two types of antihypertensive pills each day. |
| - Do not have any heart disease and history of heart surgery; do not have diabetes (36). |
| - Do not have joints and musculoskeletal disorders. |
| - Do not have a history of doing regular exercise training and in the six months leading up to the study. |
| Exclusion Criteria |
| - Having reduced willingness to take part and continue exercise sessions (lost 25% of the training program in the gym or home) (n = 8) |
| - Medical issues (n = 4) |
| - Occurring training-related injuries (n = 2) |
Inclusion and Exclusion Criteria for the Study
In this study, individual randomization units were employed. For generating random sequences, online randomization via permuted block randomization (https://www.randomization.com) with block sizes of 3 and 9 was utilized to create allocation codes. Allocation concealment was ensured through the use of sequentially numbered, sealed, opaque envelopes. In this single-blinded parallel clinical trial, the subjects were randomly allocated to two experimental conditions (GBT and HBT, n = 20 per group) and one control (CR) condition (CR, n = 20) (Figure 1). Due to the study's nature, blinding was not feasible for intervention practitioners and participants. However, other researchers who were unaware of group assignments conducted all laboratory and statistical evaluations.
It should be noted that the total number of samples required was calculated to be 57 subjects using the free trial version of the PASS 2021 software [based on fibrinogen: Power (0.80), alpha (0.05), standard deviation (SD) (17), effect size (0.42), SD of the mean (7.19), and based on apelin: Power (0.81), alpha (0.05), SD (19), effect size (0.43), SD of the mean (8.16)]. However, with the consideration of the possibility of dropping out due to exclusion criteria (Box 1), 60 subjects were selected. Because of exclusion criteria, 14 participants were not included in the final statistical analysis.
The experimental groups underwent 10 weeks of gym and at-home training, while the CR group received no training intervention. During the study, all participants were required to maintain their daily routines and take BP-lowering drugs as recommended by their medical practitioner. Additionally, to decrease dietary variability among groups, all participants were given a booklet on dietary counseling based on the Dietary Approaches to Stop Hypertension (DASH) (37).
3.2. Exercise Training Protocols
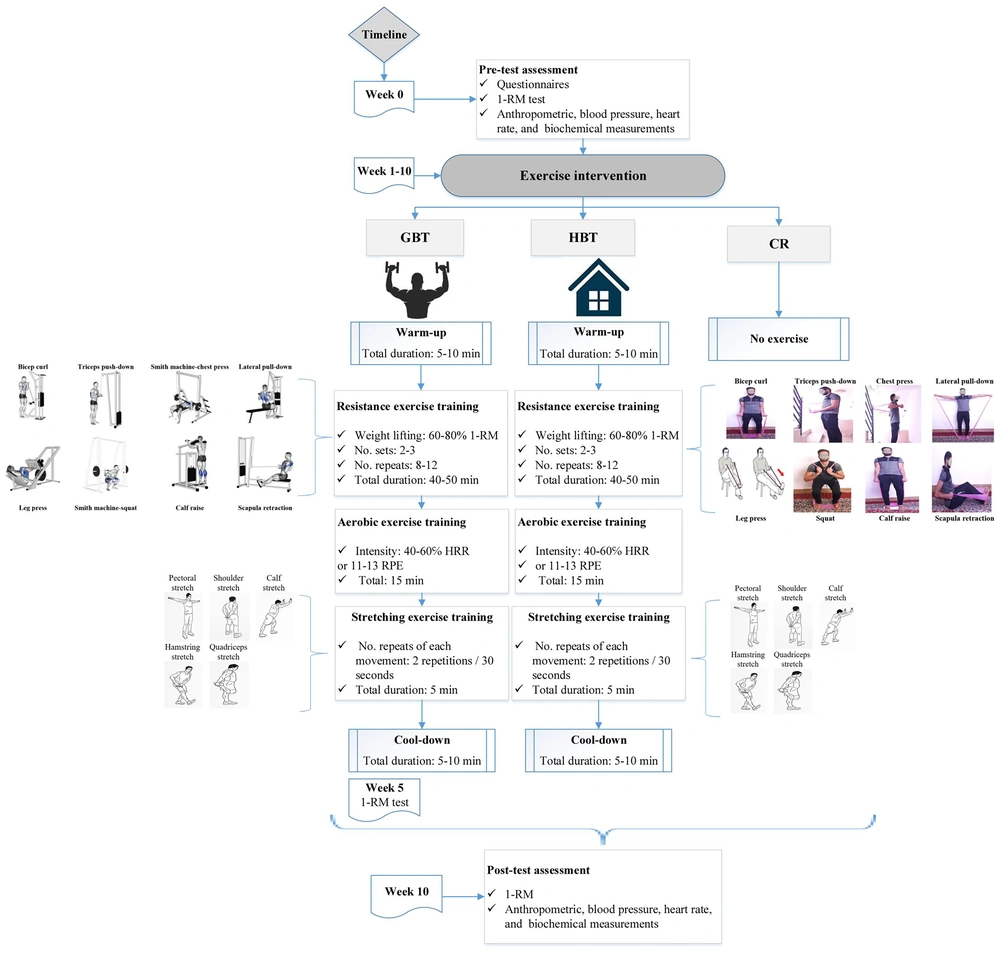
A 10-week, individualized, combined training protocol consisting of resistance, aerobic, and ST was defined based on the guidelines of the American College of Sports Medicine in frequency, intensity, type, and time (FITT principle) for hypertensive patients (38). These protocols were carried out in four consecutive sessions per week (70 - 85 minutes per session) in the gym and at home from about 5:00 to 6:30 p.m. (Figure 2). Before and after the exercise training, the participants performed general warm-ups and cool-downs based on static stretching. An exercise trainer and a nurse supervised and guided all training sessions to manage probable acute medical issues resulting from exercise training. During the study, all participants in the CR group were given instructions to carry out their daily routine and follow their physician's prescription for antihypertensive medications.
3.2.1. Gym-Based Training
First, during three familiarization sessions on non-consecutive days, those assigned to the GBT group were introduced to the concept of a one-repetition maximum (1-RM) test and exercise training prescription. Then, according to the Brzycki formula [1-RM = Weight lifted (kg) / (0.0278 - (0.0278 × Number of repetitions))] (39), the 1-RM of the target muscle groups was calculated twice: At baseline and at the end of the fifth week. Each training session started with resistance training (RT; 40 - 50 minutes) and included eight exercises (Figure 2) for the upper- and lower-body muscles (40) utilizing pin-loaded strength equipment (Technogym, Gambettola, Italy), at an intensity of 60 - 80% 1-RM, with 8 - 12 repetitions, and 2 - 3 sets based on the overload principle, along with one-minute rest periods between sets and two minutes between movements.
The AT component (15 minutes) consisted of using a treadmill or a stationary bike (40), which was carried out with an intensity of 40 - 60% heart rate reserve (HRR) or 11 - 13 on Borg’s Rating Of Perceived Exertion (RPE). The ST (5 minutes) consisted of a static stretch targeting both the upper and lower body muscles (including quadriceps, hamstring, calf, pectoral, and shoulder) (40) that was performed for 30 seconds with two repetitions for each movement (Figure 2). To ensure this, a booklet, including GBT prescriptions and the main topics that were mentioned in the familiarization sessions, was also given to the participants.
3.2.2. Home-Based Training
Participants who were assigned to the HBT group carried out a combined training prescription in the home environment, where specific muscle groups, volume, and intensity of training were similar to that of the GBT protocol (Figure 2). The only exception was RT, which used elastic bands (Thera-Band 038, Guangdong, China). During the first five weeks, participants trained with a light load pink elastic band (12 - 14 RPE or approximately 60 - 75% 1-RM), and a medium load blue elastic band (14 - 15 RPE or approximately 75 - 80% 1-RM) was used during the second five weeks. If AT gear (a treadmill or a stationary bike) was not available to participants at home, walking outdoors or in the community could be substituted while maintaining the intensity of the training.
Throughout the 10-week duration of the HBT, participants were supervised via the Telegram application on Sundays, Mondays, and Tuesdays, along with 10 weekly phone calls lasting around 10 - 15 minutes each on Wednesdays. The total follow-up time for each participant in the HBT group was equal to the time spent monitoring each participant in the GBT group. Additionally, the trainer captured six instructional videos demonstrating the correct performance of the HBT, which were then provided to the participants.
3.3. Data Collection
3.3.1. Questionnaires
At the pre-test, all participants completed a questionnaire on clinical and medical history created by the investigator, as well as a valid Persian version of the Baecke Habitual Physical Activity Questionnaire (BHPAQ) (41).
3.3.2. Height, Body Weight, and Body Mass Index Measurements
The pre-test height of the participants was measured using a BIKI 200 device (Jawon Medical, Seoul, South Korea, measured to 0.1 cm). Body weight and BMI were measured using a bioelectrical impedance analysis machine, IOI 353 (Jawon Medical, Seoul, South Korea), during both the pre-intervention and post-intervention periods.
3.3.3. Blood Pressure, Resting Heart Rate, and Aerobic Training Intensity Measurements
Systolic and diastolic BP were measured on the right arm after a 15-minute rest in a sitting position using a sphygmomanometer (ERKA D-83646 Bad Tölz, Germany) as per the guidelines provided by the American Heart Association (AHA) (42) at both the pre-test and post-test. These measurements were conducted by the same nurse during the evening hours (6:00 - 7:00 p.m.). The calculation of the maximal heart rate (MHR) was done using the formula: Maximal heart rate equals 220 minus the participant’s age (43). Participants were also instructed to estimate their resting heart rate (RHR) in the morning before rising from bed using an accurate hand-held monitor. The HRR is calculated as the difference between the MHR and the RHR. The target heart rate (THR) is then calculated using the Karvonen formula (44) as follows: Target heart rate = [(% intensity of AT × HRR) + RHR]. It should be noted that whole-body RPE (6 - 20) Scale and THR were applicable to assess the AT intensity.
3.3.4. Blood Sampling and Biochemical Assays for Superoxide Dismutase, Fibrinogen, Plasminogen Activator Inhibitor-1, Apelin, and Albumin
For the biochemical measurements, vein samples of 5 mL were collected using venoject tubes containing ethylenediaminetetraacetic acid (Hebei Xinle Sci&Tech Co., Ltd, China). These samples were taken a full day before the training began and two days after it concluded (with 12 hours of fasting, at 7:00 - 8:00 a.m.), while the participant was rested for 15 minutes sitting on a chair before sampling. The centrifuge was operated at 3000 rpm for 15 minutes, within a temperature range of 2 - 8°C, immediately to remove the plasma from the blood samples. The obtained plasma was then stored within individual microtubes (maintained at -80°C) until required.
The SOD activity was measured utilizing a quantitative analysis kit designed for human specimens (manufactured by ZellBio GmbH, located in Ulm, Germany, with an intra-assay precision coefficient of variation (CV %) of 3.4 and a sensitivity of 1 U/mL) by colorimetric method. The concentrations of fibrinogen, PAI-1, apelin, and albumin in the plasma (with an intra-assay precision CV% of 6.2, 5.2, 5.9, and 1.6 respectively, and sensitivity of 0.08 mg/mL, 0.3 AU/mL, 18 pg/mL, and 0.1 g/dL respectively) were determined using ELISA kits supplied by ZellBio GmbH, based in Ulm, Germany. All the kits were sourced from Padgin Teb Company, located in Iran.
3.4. Statistical Analysis
All statistical analyses were performed using version 23 of IBM SPSS Statistics for Windows. Mean ± SD as descriptive statistics was used to report each variable's findings. We examined the presuppositions of a one-way analysis of covariance (ANCOVA), which encompass the normal distribution of residuals (evaluated using the Shapiro-Wilk test), uniformity of error variances (assessed by Levene's test), consistency of regression gradients, and uniformity of regression line inclinations. Where all statistical assumptions were met, ANCOVA (SOD, fibrinogen, albumin, and FAR) and Bonferroni post-hoc tests were used for comparing the means among groups. In cases where the data did not follow a normal distribution, they were transformed using a base-10 logarithm for subsequent analysis. The ANCOVA analysis took into account the pre-test values as a covariate.
For non-normally distributed data (PAI-1 and apelin), the nonparametric Kruskal-Wallis test was used to compare the means among groups. Additionally, the Pearson correlation coefficient was used to measure probable associations between variables. P-values less than 0.05 were considered statistically meaningful. Graphs were produced using GraphPad Prism software (version 8).
4. Results
According to our analysis, no significant difference (P > 0.05) was found among groups in the initial attributes of participants (Table 1). We also evaluated adherence to the interventions. During the 10-week period, which required 70% compliance, a minimum of 30 sessions needed to be completed for inclusion in the final analysis. The mean total training sessions completed during the 10 weeks of the GBT and HBT were 33 sessions (83%) and 36 sessions (90%), respectively.
| Variables | CR (N = 15) | GBT (N = 16) | HBT (N = 15) | P-Value |
|---|---|---|---|---|
| Anthropometric items | ||||
| Age (y) | 46.60 ± 9.57 | 50.44 ± 9.33 | 46.00 ± 10.14 | 0.38 |
| BW (kg) | 85.12 ± 15.93 | 89.53 ± 18.83 | 86.46 ± 11.73 | 0.73 |
| BMI (kg/m2) | 28.98 ± 4.10 | 30.98 ± 5.82 | 30.17 ± 3.26 | 0.47 |
| Hemodynamics values | ||||
| Systolic BP (mmHg) | 132.86 ± 12.54 | 144.50 ± 18.63 | 134.20 ± 9.82 | 0.06 |
| Diastolic BP (mmHg) | 84.00 ± 8.63 | 86.50 ± 9.22 | 83.40 ± 6.75 | 0.54 |
| HRR (BPM) | 106.00 ± 8.20 | 98.50 ± 14.38 | 101.46 ± 11.01 | 0.20 |
| Questionnaire | ||||
| BHPAQ (score) | 7.43 ± 0.94 | 7.74 ± 0.85 | 7.50 ± 0.91 | 0.59 |
| Antihypertensive medication therapy (%) | ||||
| Monotherapy | 73.30 | 93.70 | 53.30 | - |
| Two‐drug therapy | 6.7 | 25 | - | - |
Initial Characteristics of Participants
4.1. Biochemical Outcomes
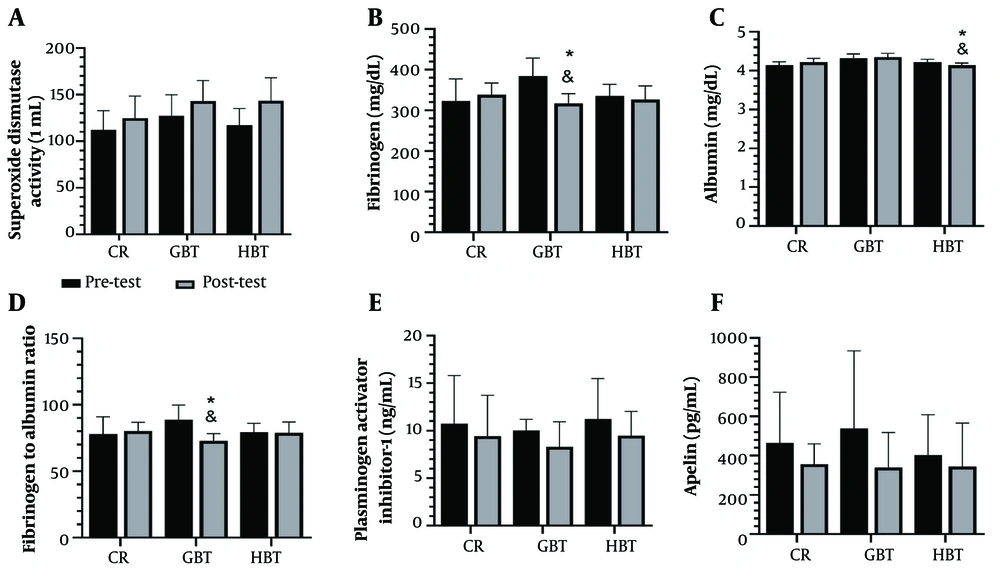
The results illustrated in Figure 3 show that the plasma levels of fibrinogen (Figure 3B) and FAR (Figure 3D) decreased significantly after GBT compared with the HBT (P = 0.02 and P = 0.001, respectively) and CR group (P < 0.001 for both comparisons). Furthermore, the plasma level of albumin decreased significantly after HBT compared with the GBT (P < 0.001) and CR group (P = 0.03) (Figure 3C). Additionally, there was a tendency for increased albumin following GBT compared with the CR group (P = 0.06). Interestingly, no significant differences were found in the plasma levels of SOD activity (Figure 3A), PAI-1 (Figure 3E), and apelin (Figure 3F) between the three groups (P = 0.12, P = 0.79, and P = 0.09, respectively) at post-test.
The plasma levels of superoxide dismutase (SOD) activity (A), fibrinogen (B), albumin (C), fibrinogen to albumin ratio (FAR) (D), plasminogen activator inhibitor-1 (PAI-1) (E), and apelin (F) after 10 weeks of gym-based training (GBT) and home-based training (HBT); (CR, control group; * significant difference compared to the CR (P < 0.05), and significant difference between the GBT and HBT groups).
4.2. Association Between Systolic Blood Pressure, Diastolic Blood Pressure, and Biochemical Values
In Table 2, Pearson correlation analysis demonstrated that systolic BP was negatively correlated with fibrinogen (r = -0.32, P = 0.02) and FAR (r = -0.33, P = 0.02) in hypertensive men after the 10 weeks of GBT and HBT, while no correlation was observed in the pre-test. Additionally, diastolic BP was positively correlated with SOD (r = 0.29, P = 0.04) in the pre-test. When we analyzed all data (pre and post-tests together), systolic BP was positively correlated with PAI-1 (r = 0.26, P = 0.01).
| Variables | Pre-tests Data (N = 46) | Post-tests Data (N = 46) | All Data (N = 92) | |||
|---|---|---|---|---|---|---|
| Systolic BP | Diastolic BP | Systolic BP | Diastolic BP | Systolic BP | Diastolic BP | |
| SOD (U/mL) | 0.08 (0.58) | 0.29 (0.04) b | 0.23 (0.11) | 0.21 (0.16) | 0.03 (0.73) | 0.06 (0.51) |
| Fibrinogen (mg/dL) | 0.06 (0.67) | 0.07 (0.63) | -0.32 (0.02) b | -0.22 (0.12) | 0.05 (0.63) | 0.07 (0.50) |
| Albumin (g/dL) | 0.22 (0.14) | 0.07 (0.64) | 0.05 (0.69) | -0.03 (0.82) | 0.13 (0.19) | 0.01 (0.91) |
| FAR (mg/dL) | 0.02 (0.89) | 0.05 (0.73) | -0.33 (0.02) b | -0.20 (0.16) | 0.01 (0.88) | 0.06 (0.56) |
| PAI-1 (AU/mL) | 0.19 (0.20) | -0.02 (0.86) | 0.27 (0.06) | 0.06 (0.67) | 0.26 (0.01) b | 0.10 (0.32) |
| Apelin (pg/mL) | 0.10 (0.47) | -0.07 (0.64) | 0.24 (0.10) | -0.03 (0.83) | 0.20 (0.053) | 0.05 (0.62) |
Pearson Correlation Between Systolic Blood Pressure, Diastolic Blood Pressure, and Biochemical Values a
5. Discussion
Our findings suggest that GBT led to more pronounced improvements in certain cardiovascular biomarkers, particularly fibrinogen and FAR, compared to HBT. These results underline the potential superiority of supervised exercise interventions in modulating cardiovascular risk factors in hypertensive men. The present study results showed that 10 weeks of GBT resulted in a significant reduction in plasma fibrinogen levels in men with primary hypertension. Researchers have suggested various mechanisms as factors affecting changes in plasma fibrinogen concentration, including increased neurotransmitter and catecholamine transporters such as epinephrine and norepinephrine, changes in fibrinolytic system activity, decreased hepatic fibrinogen production, changes in plasma volume, lipid profile, and BMI (45, 46).
Consistent with our findings, an eight-week combined training (endurance-intermittent resistance and endurance-continuous resistance) led to a significant increase in high-density lipoprotein cholesterol (HDL-C) levels and a significant decrease in plasma levels of fibrinogen, low-density lipoprotein cholesterol (LDL-C), total cholesterol (TC), and triglycerides (TG) in both intervention groups (47). A study found that an off-site walking exercise program reduces body fat and the protein interleukin-6, which lowers fibrinogen levels. Regular AT further decreases fibrinogen by reducing catecholamine stimulation and increasing blood flow and volume (48). Fibrinogen may be influenced by sympathetic nervous system activity and individuals’ lipid profiles (49). Our colleagues, in a separate study that used the same protocol and samples as the current study, found that GBT resulted in decreased plasma levels of TC and LDL-C and increased insulin sensitivity (34). Therefore, the decrease in fibrinogen levels observed in this study can be attributed to the improvement in the subjects' lipid profiles. Additionally, our separate study, which used the same protocol and samples as the current study, did not observe a significant change in the body fat percentage of the subjects (33). Therefore, the decrease in fibrinogen levels seen in the current study cannot be attributed to changes in body fat percentage. This suggests that the lack of a significant effect of HBT on improving fibrinogen plasma levels could be due to factors such as a smaller sample size (due to subject dropout), inability to perform motor skills correctly, or lack of direct supervision by the trainer on the correct execution of exercise movements. These findings suggest that supervision, exercise intensity, and protocol adherence may play a critical role in modulating fibrinogen levels in hypertensive individuals.
In this study, FAR was significantly lower in the GBT group compared to the HBT and CR groups. Additionally, the plasma level of albumin was lower in the HBT compared to the GBT group. Inconsistent with the present results, Bari et al. reported that there was no significant difference in serum albumin after a 12-week aerobic exercise program (50). They proposed that exercise leads to a redistribution of albumin from the interstitial region to the intravascular region, which increases plasma albumin levels during the first hour of recovery. This increase is likely due to enhanced lymph flow and muscle pumping during and after exercise. However, these effects are temporary and should return to normal within a few hours after exercise. If there are no other compensatory mechanisms, the spike in blood albumin levels following exercise is expected to revert to normal within 24 hours. In our study, the observed lower plasma albumin level in the HBT group may be related to differences in exercise modality, training environment, and possibly to variations in acute albumin redistribution and recovery timing.
The results of the present study showed that 10 weeks of GBT and HBT did not significantly change the level of SOD enzyme activity in men with primary hypertension. However, an upward trend in SOD activity was observed, with percentage changes in the post-test compared to the pre-test as follows: Fourteen point seven five percent in the GBT group, 23.67% in the HBT group, and 13.86% in the CR group. In line with our findings, a 10-week combined resistance and AT study involving postmenopausal hypertensive women and those with normal BP reported no significant improvement in salivary oxidative stress markers, suggesting that factors such as exercise duration and effective BP control (especially in patients using antihypertensive drugs) may play a crucial role in obtaining significant results (51).
In contrast, following eight weeks of elastic-band RT, a significant increase in SOD activity and attenuation of oxidative stress indices was observed in healthy men (52). Additionally, it has been reported that SOD enzyme activity may not be highly sensitive to RT intensity and can improve regardless of sequence (53), while AT appears to exert superior effects in combating oxidative stress (54). From a biochemical perspective, enzyme concentration itself can influence enzyme kinetics and reaction efficiency (55). Although no statistically significant change was detected in SOD activity in our study, the concentration of the enzyme may have changed, affecting its antioxidant function. In our study, the modest increase in SOD activity observed after HBT may be attributed to the relatively short intervention duration and moderate AT intensity.
Despite impaired apelin levels and NO bioavailability in older hypertensive patients, exercise training is considered an effective intervention for treating hypertension (56). However, in the present study, the plasma level of apelin remained unchanged after GBT and HBT in men with primary hypertension. In contrast, another study using combined training (resistance; 60 - 70% 1-RM + aerobic; running on a treadmill, 60 - 75% HRR) resulted in a significant decrease in the apelin level and improved body composition indices in middle-aged women (57). Similarly, isocaloric interventions (combining training and nutrition) reduced plasma apelin 36 and HOMA-IR levels in overweight men (58). Conversely, obese adult women showed a significant increase in apelin concentration after descending stair walking training (59).
Regarding the mechanisms underlying exercise training's effects on apelin, the translocation of GLUT-4 to cell membranes is mediated by AT, as shown by animal studies, potentially decreasing apelin gene expression. Conversely, the level of apelin may decrease through an increase in lipoprotein lipase activity in muscles and a decrease in insulin resistance due to RT-induced fatty acid oxidation (56). Furthermore, nine weeks of swimming training attenuated hypertension pathogenesis and reversed the cardiovascular apelin/APJ system downregulation induced by hypertension. This suggests that exercise training’s beneficial impact on hypertension may involve upregulation of the cardiovascular apelin/APJ system (60). A study declared that by increasing apelin and nitrite/nitrate plasma levels, high-intensity interval training may be effective in reducing BP (56).
Despite this, there is inconsistency in the literature regarding the effects of exercise training on apelin concentrations in hypertensive individuals. Our present study included stretching exercises, which are beneficial for improving flexibility and reducing BP. The inconsistent results may be due to differences in study design, training duration, stretching exercise volume, sample size, genetic characteristics, nutritional status, age variations, and exercise modalities. These findings underscore the need for training protocols with greater intensity or duration to potentially influence apelin concentrations in hypertensive individuals.
The AT, particularly at higher intensity, appears to reduce PAI-1 levels more effectively than RT. Generally, increased levels of physical activity are associated with lower circulating PAI-1 levels. During acute bouts of vigorous exercise, the risk of thrombotic events increases, but after regular high-intensity training, platelet aggregation caused by exercise may be desensitized, and by decreasing PAI-1 response and increasing tPA, it can up-regulate fibrinolytic potential (61). One year of lifestyle modification, including increased moderate-intensity training, decreased caloric and saturated fat intake, and changes in the composition of macronutrients, resulted in a significant reduction in PAI-1 levels. Furthermore, improvements in cardiorespiratory fitness, HDL-C, and glucose control were associated with lower PAI-1 levels, independent of adiposity (62).
In the present study, the plasma level of PAI-1 remained unchanged after the exercise training intervention. Considering the limited studies on the effect of combined training (resistance, aerobic, and stretching) in the gym and at home on PAI-1, it seems that the lack of significant reduction in PAI-1 levels in the current study could be due to the short duration and moderate intensity of the aerobic and RT, or the inclusion of a stretching component in the training protocol. Moreover, limitations such as small sample size due to attrition, potential motor execution errors, and absence of direct supervision — particularly in the HBT group — may have influenced the outcomes. These findings suggest that higher exercise intensity, improved supervision, and refined program design may be necessary to elicit favorable changes in fibrinolytic markers like PAI-1 in hypertensive individuals (61).
5.1. Conclusions
In conclusion, GBT and HBT appeared to be effective in modulating certain cardiovascular risk factors in men with primary hypertension. The GBT, under the direct supervision of coaches, may serve as a more effective approach for optimizing cardiovascular health outcomes in this population. Meanwhile, HBT may represent a valuable and accessible alternative for individuals who face physical, psychological, or socio-economic barriers to attending gym-based programs. Future studies are warranted to further explore optimized training protocols and supervision methods to potentially maximize cardiovascular benefits in diverse patient populations.
5.2. Limitations
The current research has several limitations that should be considered in future research. It is worth noting that in the current study, there were no other samples available to replace those who dropped out. Therefore, the results obtained were based on a smaller sample size than calculated, which may have affected the validity of the results. For more accurate findings in future studies, a larger sample size is recommended. Furthermore, assuming the possibility of insufficient supervision over the execution of HBT, it is suggested that in future studies, various other remote supervision methods for the execution of relevant exercise protocols should be examined.