1. Background
Diabetes mellitus is recognized as the most prevalent endocrine and metabolic disorder affecting children and adolescents. This condition is marked by hyperglycemia, which arises when the beta cells fail to function effectively or are destroyed. Type 1 diabetes (T1DM) mellitus is a persistent autoimmune condition characterized by the destruction of beta cells located in the islets of Langerhans (1). Childhood-onset diabetes mellitus is linked to an increased risk of early mortality, particularly in individuals exhibiting elevated levels of glycated hemoglobin (HbA1c) (2). The T1DM is associated with a heightened risk of atherosclerosis and a greater incidence of cardiovascular disease (CVD), making CVD the leading cause of premature mortality and disability within this demographic (3).
Numerous circulating microRNAs (miRNAs) have been consistently identified as having differential abundance in patients with diabetes mellitus compared to healthy individuals. A study examining the serum miRNA expression profiles in children who have recently been diagnosed with T1DM revealed 12 miRNAs that were significantly up-regulated, including miR-24, miR-26a, miR-27b, miR-27a, miR-29a, miR-25, miR-152, miR-30a-5p, miR-200, miR-148a, miR-181a, and miR-204 (4, 5). Some of these miRNAs are linked to the regulation of glycemic control and the restoration of pancreatic β-cell function, with miR-181a also noted for its up-regulation in T1DM patients (5).
Moreover, research has shown that levels of thioredoxin-interacting protein (TXNIP), which plays a role in regulating the redox state of β cells, are elevated in type 2 diabetes mellitus (5, 6). This elevation leads to an increased expression of miR-204, which subsequently inhibits insulin secretion by directly targeting the MAF transcription factor A (MafA), a key positive regulator of the insulin gene, thereby promoting the degradation of MafA. Numerous studies have indicated that specific miRNAs can directly influence components of the insulin signaling pathway. For instance, miR-181 can adversely impact insulin signaling by indirectly modulating the insulin receptor through the regulation of protein tyrosine phosphatases, which are responsible for dephosphorylating tyrosine residues in the cytoplasmic domain associated with insulin resistance (5, 7). Dyslipidemia is a metabolic condition commonly found in young individuals with T1DM, which elevates the risk of CVD (8).
Participation in exercise training can improve the metabolic profile, enhance cardiovascular fitness, and improve insulin sensitivity, thereby lowering the mortality risk in children diagnosed with T1DM, as the health benefits associated with exercise can last into adulthood (9). Current clinical guidelines regarding exercise for adolescents with T1DM are still being developed, as they primarily draw from research focused on patients with type 2 diabetes. Physical activity serves as a crucial factor influencing physical fitness, encompassing various components such as aerobic capacity and muscular strength, both of which are vital indicators of overall health (10, 11). However, there are some conflicting results regarding the expression of miR-204 and miR-181 as potential biomarkers of T1D (12, 13).
Given the positive impact of physical activity on lipid profiles, body composition, and glucose regulation in adolescents with T1D, it is reasonable to anticipate that regular physical activity enhances metabolic and cardiorespiratory fitness in young individuals, helps prevent weight gain, and serves as a crucial lifestyle factor that has been shown to diminish the severity and risk of CVDs and other chronic non-communicable diseases throughout life (5).
2. Objectives
This study aims to assess the expression of miRNA-181 and miRNA-204 and to explore the relationship between these miRNAs and various clinical and physical fitness parameters in children with T1D. Additionally, the potential of miRNA-181 and miRNA-204 as biomarkers for assessing the residual function of pancreatic beta cells will be analyzed.
3. Methods
This is a quasi-experimental clinical trial study conducted in a crossover design. A total of 12 diabetic children, who received treatment under the guidance of a specialist, were initially randomly divided into two groups, with each group comprising six participants. The initial group will commence with aerobic exercise (AE), which will be succeeded by a one-month washout period, after which resistance exercise (RE) will take place. Conversely, the second group will initiate the study with RE, followed by a one-month washout, and subsequently engage in AE. Following the initial phase of interventions, one individual from the AE group and two individuals from the RE group were removed due to excessive absenteeism from training, and the interventions proceeded: The AE (n = 9, age = 12.20 ± 1 years), The RE (n = 9, age = 12.20 ± 1 years) (7 boys and 2 girls).
None of the subjects had engaged in any consistent exercise regimen over the previous two years, and a minimum of two years had elapsed since their diagnosis of T1DM. All participants were monitored by two specialist physicians and received insulin injections without the use of an insulin pump. Their dietary regimen adhered to the guidelines and directives provided by their treating physician, making it virtually unfeasible for the researcher and their colleagues to oversee their dietary intake.
According to the health survey, all participants reported that they had not encountered any illness or physical injury that could impede their capacity to exercise. Participants were chosen from eligible patients through a non-random selection process. Prior to their involvement, the parents of the subjects filled out a general health screening questionnaire and provided written consent for their voluntary participation in the study. The participants were categorized into two groups, each consisting of 9 individuals. In the AE group, training was organized two times per week in a home training format, with careful monitoring of the exercise's intensity, duration, and load progression.
Furthermore, the participants engaged in their exercises weekly under the guidance of the researcher to ensure the correct performance of the exercises, as well as to manage the intensity and pace of their independent training sessions at home (twice a week). They documented their exercises and submitted videos of all activities to the research team every week. Resistance training was conducted three times weekly and was structured as home-based RE, utilizing the individual's body weight as resistance. The parameters of intensity, duration, and effort improvements within the training sessions were meticulously tracked. To facilitate this, participants engaged in their exercises with the supervision of a researcher once per week, while documenting their home practice sessions (twice weekly). The principle of progressive overload was applied by increasing the number of training sets once every two weeks and also increasing the time spent performing the exercises. Table 1 provides a summary of the exercise protocols for AE and RE groups.
| Variables | Time/Repetition | Sets | Recovery Time (s) |
|---|---|---|---|
| AE protocol | |||
| Aerobic step | 20 - 60 d | 2 - 4 | 90 |
| Mountain climber | 30 - 120 d | 2 - 4 | 90 |
| Jumping Jack | 10 - 20 d | 2 - 4 | 90 |
| Heel-to-butt kick | 10 - 20 d | 2 - 4 | 90 |
| Running in place | 30 - 60 d | 2 - 4 | 90 |
| RE protocol | |||
| Half squat | 10 - 15 e | 2 - 4 | 60 |
| Modified crunch | 10 - 15 e | 2 - 4 | 60 |
| Plank-side plank | 20 - 60 d | 2 - 4 | 60 |
| Push-up | 10 - 30 e | 2 - 4 | 60 |
| Lunge | 10 - 30 e | 2 - 4 | 60 |
This research was conducted with the approval of the Research Ethics Committee of University of Isfahan, with the number IR.UI.REC.1403.026 and it is registered in the Clinical Research Center of Iran with the number IRCT20240516061815N1.
3.1. Measurement of Physical Fitness Factors and Laboratory Methods
To assess various physical fitness components, the following methods were utilized: The endurance of the abdominal muscles was measured through a sit-up test conducted over 60 seconds; the endurance of the shoulder muscles was evaluated via a press-up test, also within 60 seconds; the flexibility of the trunk muscles was assessed through the sit and reach test; the static strength of hand muscles was measured using a dynamometer device; and the body fat percentage was determined by a body composition analyzer device (In-Body Company-Korea). Additionally, the 20-meter shuttle run (20-MST) test, along with the corresponding equation, was applied to ascertain the maximum oxygen consumption (14): VO2peak = 61.1 - 2.20 gender - 0.462 age - 0.862 Body Mass Index (BMI) + 0.192 TL. Where TL is the total number of laps covered in the 20-MST, gender is coded as female: One and male: None. The BMI was determined by dividing the weight in kilograms (kg) by the height in square meters (m2) of the participants. Blood samples along with Physiological Index measurements were collected 48 hours prior to the initial training session and were repeated 48 hours following the final training session.
3.2. Micro-RNA Extraction and Quantification
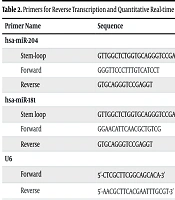
The miRNAs were extracted from serum using the miRNeasy kit (Qiagen, Germany), following the manufacturer’s protocol. Primers for reverse transcription and quantitative real-time PCR analysis of the miRNAs, with U6 as a housekeeping gene, were designed using the miRNA design tool developed by Astrid Research. The sequences of the primers are listed in Table 2.
| Primer Name | Sequence |
|---|---|
| hsa-miR-204 | |
| Stem-loop | GTTGGCTCTGGTGCAGGGTCCGAGGTATTCGCACCAGAGCCAACAGGCAT |
| Forward | GGGTTCCCTTTGTCATCCT |
| Reverse | GTGCAGGGTCCGAGGT |
| hsa-miR-181 | |
| Stem loop | GTTGGCTCTGGTGCAGGGTCCGAGGTATTCGCACCAGAGCCAACACTCAC |
| Forward | GGAACATTCAACGCTGTCG |
| Reverse | GTGCAGGGTCCGAGGT |
| U6 | |
| Forward | 5′-CTCGCTTCGGCAGCACA-3′ |
| Reverse | 5′-AACGCTTCACGAATTTGCGT-3′ |
Primers for Reverse Transcription and Quantitative Real-time PCR Analysis
The levels of hsa-miR-204 and hsa-miR-181 were quantified using the SYBR Green method. Reverse transcription was carried out with miRNA-specific stem-loop primers and the RScript™ miRNA cDNA Synthesis Kit (BIO-HELIX, Taiwan) on a Biolab thermal cycler, following the manufacturer's instructions. Triplicate quantitative real-time PCR was performed using the miRNA qPCR Kit (Abnova, Taiwan) on the Rotor-Gene 6000 real-time PCR system (Corbett Research, Australia). The resulting data were analyzed using fold change and the ΔΔCt methods with REST software.
3.3. Blood Sample Lipid Profile Changes
Changes in lipid profiles, including triglycerides (TG), total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), fasting blood sugar (FBS), and insulin, were measured using the Delta Darman Part Human commercial kit (manufactured in Iran). Blood samples (10 mL) were obtained from the cubital vein by a qualified professional 48 hours prior to and following the intervention. The samples were allowed to remain at room temperature for a period of 10 to 15 minutes before undergoing centrifugation for 10 minutes at a speed of 3000 rpm (15). The supernatants were meticulously extracted and subsequently frozen at -80°C for future analysis. Participants were instructed to refrain from medication and strenuous physical activities for 72 hours before sampling, and these conditions were maintained for the last assessment at the end of week eight of the intervention.
3.4. Statistical Analysis
A preliminary calculation of the sample size using G-Power 3.1.9.2 software revealed that at least 28 participants were required for the study, based on an alpha level of 0.05, a power of 0.80, and an effect size of 0.4. Descriptive statistics, including the mean and standard deviation, were presented, and the assumptions of homogeneity of variance and normality of data were assessed using Levene’s and Shapiro-Wilk tests, respectively. The effects of the training interventions on dependent variables were analyzed using analysis of covariance (ANCOVA) statistics. All statistical analyses were performed using SPSS version 26, with a significance level set at P < 0.05.
4. Results
Mean and standard deviation values of measured variables such as physical fitness components, as well as metabolic variables measured in two phases of the pre-test and post-test, along with the percentage of changes noted after eight weeks of exercise training, are presented in Tables 3 and 4.
| Measured Variables | AE | Percent Changes | RE | Percent Changes |
|---|---|---|---|---|
| Weight (kg) | -1.99 | -1.52 | ||
| Pre | 50.67 ± 14.8 | 51.44 ± 15.7 | ||
| Post | 49.66 ± 15.0 | 50.66 ± 14.8 | ||
| miR-181 (fold change) | 2.2 | -1.61 | ||
| Pre | 25.87 ± 0.85 | 26.13 ± 1.4 | ||
| Post | 26.44 ± 0.43 | 25.71 ± 0.77 | ||
| miR-204 (fold change) | 2.55 | 3.46 | ||
| Pre | 27.86 ± 1.39 | 26.85 ± 1.05 | ||
| Post | 28.57 ± 1.73 | 27.78 ± 1.4 | ||
| Insulin (µIu/mL) | 45.8 | 41.79 | ||
| Pre | 1.92 ± 0.93 | 2.01 ± 1.03 | ||
| Post | 2.80 ± 1.09 | 2.85 ± 1.22 | ||
| Hemoglobin A1C (%) | 7.2 | 13.15 | ||
| Pre | 8.14 ± 1.06 | 8.06 ± 0.99 | ||
| Post | 7.55 ± 0.88 | 7.0 ± 1.09 | ||
| Fasting glucose (mg/dL) | -10.26 | -19.34 | ||
| Pre | 184.66 ± 35.36 | 182.11 ± 33.11 | ||
| Post | 165.7 ± 29.58 | 146.88 ± 36.49 | ||
| HOMA-IR | 28.03 | 16.23 | ||
| Pre | 0.906 ± 0.52 | 0.912 ± 0.47 | ||
| Post | 1.160 ± 0.50 | 1.06 ± 0.55 | ||
| LDL-C (mg/dL) | -23.23 | -30.64 | ||
| Pre | 128.67 ± 44.90 | 128.00 ± 43.40 | ||
| Post | 98.78 ± 24.83 | 88.78 ± 20.28 | ||
| HDL-C (mg/dL) | 40.49 | -7.59 | ||
| Pre | 56.11 ± 16.18 | 52.67 ± 15.46 | ||
| Post | 62.00 ± 16.52 | 56.67 ± 16.03 | ||
| Triglyceride (mg/dL) | -34.4 | -11.6 | ||
| Pre | 150.89 ± 70.41 | 159.00 ± 74.66 | ||
| Post | 105.00 ± 53.00 | 140.55 ± 72.64 | ||
| Cholesterol (mg/dL) | -18.2 | -27.06 | ||
| Pre | 217.33 ± 54.07 | 215.11 ± 52.61 | ||
| Post | 177.78 ± 44.68 | 156.89 ± 28.10 | ||
| BMI (kg/m2) | 0.01 | -1.61 | ||
| Pre | 20.8 ± 3.9 | 21.14 ± 3.4 | ||
| Post | 20.8 ± 4 | 20.8 ± 3.9 | ||
| Body fat (%) | -3.68 | -2.55 | ||
| Pre | 27.2 ± 11.8 | 27.5 ± 11.9 | ||
| Post | 26.2 ± 11.6 | 26.8 ± 11.8 | ||
| VO2peak (mL/kg/min) | 1.8 | 2.84 | ||
| Pre | 43.4 ± 3.8 | 42.2 ± 4.2 | ||
| Post | 44.2 ± 3.9 | 43.4 ± 3.8 | ||
| Hand grip (kg) | 6.4 | 13.1 | ||
| Pre | 22.61 ± 5.34 | 20.00 ± 4.38 | ||
| Post | 24.06 ± 5.32 | 22.61 ± 5.34 | ||
| Curl-up (count) | 15.4 | 18.9 | ||
| Pre | 24.22 ± 5.35 | 20.55 ± 6.18 | ||
| Post | 28.89 ± 7.74 | 24.44 ± 7.63 | ||
| Push-up (count) | 7.9 | 11.2 | ||
| Pre | 22.44 ± 5.98 | 5.98 ± 1.99 | ||
| Post | 24.22 ± 5.35 | 6.65 ± 2.21 | ||
| Sit and reach (cm) | 6.5 | 8.15 | ||
| Pre | 28.00 ± 7.44 | 25.89 ± 7.09 | ||
| Post | 29.83 ± 7.04 | 28.00 ± 7.44 |
Descriptive and Physiological Characteristics of Subjects Before and After Interventions a
| Measured Variables | t-Test | ANCOVA | ||||
|---|---|---|---|---|---|---|
| AE | RE | F | P-Value | |||
| t | P-Value | t | P-Value | |||
| miR-181 (fold change) | -1.796 | 0.115 | 0.944 | 0.373 | 6.726 | 0.020 a |
| miR-204 (fold change) | -1.249 | 0.247 | -2.877 | 0.021 a | 0.022 | 0.885 |
| Insulin (µIu/mL) | -8.931 | 0.001 b | -7.800 | 0.001 b | 0.118 | 0.736 |
| Hemoglobin A1C (%) | -4.799 | 0.001 b | -5.176 | 0.001 b | 0.005 | 0.944 |
| Fasting glucose (mg/dL) | 2.996 | 0.017 | 3.235 | 0.012 a | 1.950 | 0.183 |
| HOMA-IR | 3.864 | 0.005 b | 2.236 | 0.056 | 1.390 | 0.257 |
| LDL-C (mg/dL) | 2.377 | 0.45 | 3.663 | 0.006 b | 1.272 | 0.277 |
| HDL-C (mg/dL) | 2.157 | 0.063 | 1.409 | 0.196 | 0.321 | 0.579 |
| Triglyceride (mg/dL) | 3.855 | 0.005 b | 3.763 | 0.006 b | 6.276 | 0.024 a |
| Cholesterol (mg/dL) | 3.216 | 0.012 a | 4.617 | 0.002 a | 2.415 | 0.141 |
| BMI (kg/m2) | 0.165 | 0.837 | 1.490 | 0.175 | 1.795 | 0.200 |
| Body fat (%) | 1.993 | 0.081 | 1.216 | 0.259 | 0.174 | 0.842 |
| VO2peak (mL/kg/min) | -5.998 | 0.001 b | -3.986 | 0.004 a | 3.042 | 0.078 |
| Hand grip (kg) | 2.571 | 0.33 | 2.937 | 0.019 | 1.20 | 0.328 |
| Curl-up (count) | 3.885 | 0.005 b | 4.819 | 0.001 b | 0.100 | 0.756 |
| Push-up (count) | 2.101 | 0.069 | 5.363 | 0.001 b | 0.495 | 0.493 |
| Sit and reach (cm) | 6.633 | 0.001 b | 11.590 | 0.001 b | 0.569 | 0.462 |
The Results Related to the Statistical Analysis of Data
The main outcomes of this study were changes in the measured mi-RNAs expression, which are shown in Table 3. The values of mi-RNA-204 significantly decreased in the RE group (t = -2.877; P = 0.021) from baseline to post-test, while they remained unchanged in the AE training groups (t = 0.247; P = 0.115). Although we observed no changes in pre- to post-test values for miR-181 expression, there were significant changes in between-group comparisons (P = 0.020).
The findings of the covariance statistics for the presence of differences between exercise groups in measured variables show that, except for miR-181 and triglyceride changes, in most cases, the effects of the two exercise methods are not significantly different. Additionally, the outcomes of a paired t-test to measure within-group changes show a significant increase in insulin and a significant decrease in fasting glucose, HOMA-IR, triglyceride, and cholesterol levels in both groups after eight weeks of exercise training. Moreover, notable enhancements were recorded in the performance of sit-ups and push-ups, hand grip strength, and VO2peak levels in both training groups. The information related to the results of the dependent t-test and covariance model is presented in Table 3.
5. Discussion
Regarding the effects of two exercise training protocols on miR-204 and miR-181 levels in T1D diabetic children, the results are contradictory and limited. Our findings revealed a significant increase in miR-204 levels in the RE and AE exercise groups in within-group comparison, but there was no significant difference in miR-181 levels from pre- to post-test assessments. However, findings revealed that in between-group comparisons, AE can increase miR-181 levels in diabetic children, and RE can reduce the same miRNA in diabetic children. In a systematic review by Margaritis et al., it was reported that the levels of miR-181 were elevated in children with diabetes (16). The objective of utilizing miRNAs as biomarkers for T1D is to examine their expression patterns, facilitating early disease prediction and the identification of autoimmunity triggers before the conversion of autoantibodies. The miRNA-181 has been recognized as a crucial factor in the onset and advancement of T1D, particularly regarding T-cell regulation and islet autoimmunity. Regular physical activity, especially exercise, has been demonstrated to affect miRNA expression, which may influence processes related to T1D (17).
Although the precise mechanisms remain under exploration, certain studies indicate that alterations in miRNA expression due to exercise could adjust immune responses and possibly provide therapeutic advantages for T1D. Zhang et al. also showed that miR-181 levels in diabetic children are up-regulated (17). However, our findings do not align with these studies. miR-204 is a microRNA that is significantly abundant in human beta-cells. Some research indicates that it is released from dying beta-cells and can be detected in human serum. Additionally, studies reported that serum levels of miR-204 were increased in both children and adults diagnosed with T1D, as well as in autoantibody-positive individuals at risk, but not in those with type 2 diabetes or other autoimmune conditions. Furthermore, there was an inverse correlation between serum miR-204 levels and the remaining beta-cell function in individuals with recent-onset T1D. Therefore, serum miR-204 may represent a valuable new method for evaluating the early loss of human beta-cells associated with T1D, even before the manifestation of overt disease (18, 19). Although the ability of exercise interventions to change circulating miRNAs has been reported in several studies, the mechanism of its effect and which group will benefit the most are unclear (20). Physical activity can influence the expression of microRNA-204, which is involved in the regulation of glucose metabolism and may impact insulin sensitivity in the context of T1DM. Research indicates that exercise may elevate miR-204 levels in skeletal muscle, potentially facilitating glycolysis and enhancing glucose clearance (21). The results of our research align with earlier studies. Variations in this biomarker observed in certain studies may be influenced by a range of factors, such as diet, physical fitness, and diverse environmental influences. These factors were not controllable in the present study and should be considered in future research. It is widely accepted that the most significant enhancement in lipid profile levels correlates with decreases in BMI, body fat percentage, and total body weight. Furthermore, incorporating a diet aimed at decreasing body fat, alongside engaging in physical activity, contributes to an additional decline in lipid profile levels (20, 22). Numerous studies highlight the advantages of physical activity on metabolic parameters, demonstrating its positive effects across various intensities (23). Our results add to the growing evidence that physical activity may improve the metabolic profile, which is an important process in the treatment of T1D disease in children. Additionally, our results showed improvements in the strength and endurance of muscles, as well as an improvement in cardiovascular capacity in exercise intervention groups. Previous research has indicated that individuals with T1DM experience enhancements in VO2max, muscular strength, waist circumference, and resting heart rate as a result of engaging in regular REs and high-intensity interval training (24, 25). It is crucial to emphasize that children and adolescents diagnosed with diabetes, unlike the general population, necessitate continuous insulin therapy to achieve an ideal health status. Moreover, an overabundance of insulin administration is often associated with weight gain and abdominal obesity. Furthermore, the fear of hypoglycemia can affect their lifestyle decisions, which may result in reduced physical activity, a decline in physical health, and the development of obesity (26). In the meantime, research indicates that exercise has positive effects on regulating blood glucose levels and reducing the risk of hypoglycemia. Furthermore, study findings demonstrate that engaging in physical activity, regardless of the type, serves as an effective strategy for preventing overweight and obesity, reducing the incidence of cardiovascular disorders, and enhancing overall physical fitness (27). The results indicate that engaging in physical activities leads to an increase in the AMP/ATP ratio, which subsequently elevates the levels of AMP-activated protein kinase (AMPK) (28). This protein acts as a protective sensor that assesses the energy status of the cell. Its activation triggers mitochondrial biogenesis, encourages fatty acid oxidation, increases insulin sensitivity, and aids in glucose absorption; these conditions contribute to the reduction of body fat, enhancement of lipid profiles, and improvement of glucose metabolism (29). Furthermore, additional scientific evidence indicates that the signaling of intramuscular AMP and the activity of AMPK are directly correlated with the intensity of exercise (30). Our analysis reveals a minor elevation in high-density lipoproteins (HDLs) levels and a corresponding decline in LDL-C, as well as total cholesterol and triglyceride levels within the AE group. Furthermore, the measurements associated with HbA1c and fasting blood glucose exhibited a significant decrease, while insulin levels for both exercise interventions showed a significant increase when comparing pre- and post-test results. There is a widespread consensus on the substantial advantages of engaging in regular, low-to-moderate-intensity physical activities for the regulation of blood glucose levels and lipid profiles. Furthermore, in numerous instances, enhancements in insulin sensitivity and a decrease in the required dosage of injected insulin following various forms of exercise have been documented (10).
Lipid metabolism encompasses various interrelated pathways, including the hepatic production of very low-density lipoprotein (VLDL), the uptake of fatty acids by skeletal muscles or adipose tissue, the extrahepatic transport of cholesterol via low-density lipoproteins, and the elimination of surplus cholesterol by HDL (31). This indicates that higher HDL values are associated with a lower incidence of CVD. The HDL serves as the key lipoprotein in the process of reverse cholesterol transport. Reverse cholesterol transport is the mechanism by which excess cholesterol is removed from peripheral tissues and delivered to the liver (where it is distributed to other tissues) or bile for excretion (32). Existing findings show that regular exercise can lead to better metabolism of blood lipids by increasing HDL (31). Concerning the management of blood glucose levels, Yardley et al. recognized that engaging in strength training, whether independently or alongside AEs, can significantly influence the reduction of HbA1c levels. Nevertheless, certain researchers have reported a lack of any beneficial effect from the implementation of alternative exercise modalities (33). Our findings additionally indicated that despite the small changes in HbA1c in the RE and AE groups in within-group comparisons, the reduction of HbA1c after both exercise interventions is not significant. Recommendations regarding the appropriate type, duration, and intensity of physical activity are frequently revised by entities like the American Diabetes Association and the International Society for Pediatric and Adolescent Diabetes. It should be noted that exercise in patients with T1DM should stimulate efforts to be more active and, on the other hand, maximize the beneficial effects on health.
Our research suggests that in children diagnosed with T1DM, both AE and RE contribute to the enhancement of lipid profiles, as well as improvements in physical fitness, body composition, and glycemic control. Consequently, regular physical activity should be regarded as a crucial element in the management of T1DM, as it aids in the prevention and mitigation of cardiovascular disorder risk factors among diabetic children. One of the primary limitations of this research was its restricted sample size. Despite the acknowledgment of the significance of different sports activities in managing and aiding the treatment of T1DM, parents continue to exhibit particular apprehension regarding their children's involvement in physical activities. Furthermore, the involvement of medical professionals in this area is crucial and essential. The study's limitations, including the absence of a control group, short exercise intervention duration, and inadequate dietary control, restrict the generalizability and strength of the findings. A control group is crucial for comparison, and a longer intervention duration allows for a more robust assessment of effects. Dietary control is important for isolating the impact of exercise on the outcomes being measured.