1. Background
In living organisms, the brain serves as the hub of thought, emotion, desire, perception, curiosity, learning, memory, and behavior (1, 2). Understanding how learning and memory function has long captivated physiologists (3, 4), as these capabilities enable individuals to adapt to their surroundings, form unique personal memories, advance education and culture, and ultimately shape civilization itself (4, 5). Learning and memory represent two of the most advanced and intricate operations of the nervous system. Learning involves acquiring new information, reflected in changes in behavior, while memory is the outcome of this process, allowing us to store and recall experiences and knowledge (6, 7).
2. Methods
2.1. Characteristics of Studied Animals
The rats were divided into five groups of 13 as follows.
2.1.1. Passive Avoidance Learning Behavior Test
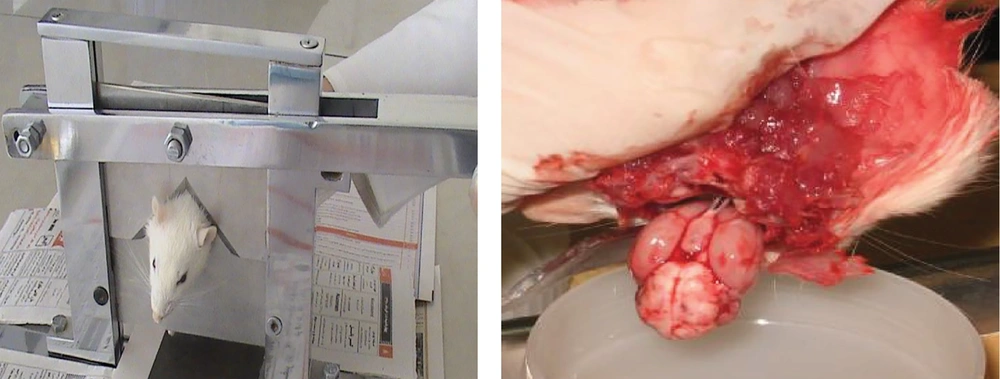
To assess passive avoidance learning behavior, researchers utilized a shuttle box apparatus designed to measure memory retention. The device consists of two interconnected chambers, one brightly lit and the other dark. Both chamber floors are lined with steel wires spaced one centimeter apart and measuring 1 - 2 millimeters in diameter (8, 9).
2.1.2. Morris Water Maze Behavioral Test
For testing purposes, the tank is conceptually divided into four equal quadrants. Within one designated quadrant, a concealed and detachable platform measuring 10 cm in diameter is positioned approximately 2 cm beneath the water surface.
2.1.3. Open Field Test
The test chamber, constructed from Plexiglas, was maintained under low-light conditions during the experiment. Each mouse was individually introduced into a corner of the box, and its behavior was recorded on video for a period of five minutes (10).
2.2. Extraction of the Hippocampus
Following the completion of behavioral testing, the animals were anesthetized using ether. Subsequently, decapitation was performed via guillotine, and the brain was promptly extracted. The isolated brain tissue was rinsed with cold 0.15 M phosphate-buffered saline (PBS), after which the hippocampal region was dissected and stored at -80°C for future analyses (Figure 1).
Stained brain tissue sections were analyzed to quantify neuronal populations within the CA1 and CA3 regions of the hippocampus. Using light microscopy, researchers examined the sections to assess neuronal diameter and determine the number of degenerated or non-viable neurons.
2.3. Data Analysis
Statistical analyses were performed using SPSS and Instat software. Results are presented as mean ± standard error of the mean (SEM). Data were evaluated through one-way and two-way analysis of variance (ANOVA).
3. Results
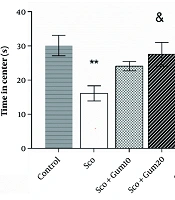
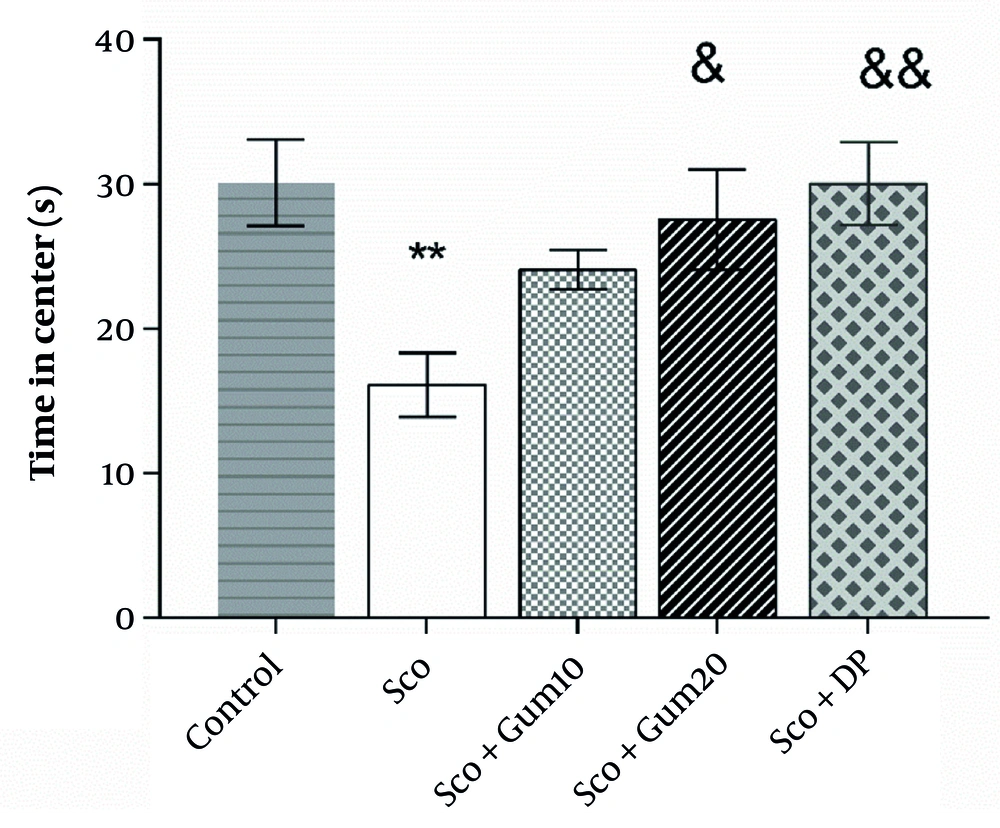
Open field test results are shown in Figure 2. As illustrated, the time spent in the central area was significantly reduced in the scopolamine group (16.10 ± 2.21 s) compared to the control group (30.09 ± 2.96 s) (P < 0.01). However, the time spent in the center (24.07 ± 1.35 s) with the injection of gummosin 10 in the scopolamine + gummosin 10 group returned to the level of control values. Additionally, in the scopolamine + gummosin 20 and Sco + DP groups, the time spent in the center (27.55 ± 3.48 s and 30.03 ± 2.84 s, respectively) was significantly lower compared to the control group (P < 0.05 and P < 0.01, respectively) (Figure 2).
The effects of Gummosin injection in memory disorder model rats on anxiety-related behaviors in the Open field test. Gummosin injection with a dose of 20 in the Sco+Gum20 group shows a significant increase in the time spent in the center compared to the scopolamine group. Data are expressed as Mean± SEM, ** P < 0.01, compared with the control group. & P < 0.05 and && P < 0.01 , compared with scopolamine group.
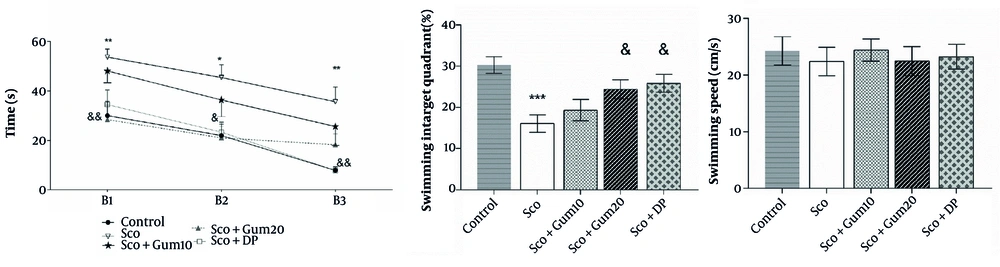
Animals’ spatial learning was assessed by the change in time spent to find the hidden platform during 12 learning phases in three blocks. As shown in Figure 3, a progressive decrease in the time to reach the hidden platform was observed in the control group. The Sco + DP group demonstrated better learning than the scopolamine group in the second and third blocks (P < 0.05 and P < 0.01, respectively) (Figure 4A). Twenty-four hours after the last learning session, spatial memory retention was assessed (Figure 4B). The retention levels in the scopolamine + gummosin 20 and Sco + DP groups reached the normal level and were equal to the control group, but were significantly higher than the scopolamine group (P < 0.05) (Figure 3B). Additionally, swimming speed was consistent across all groups (Figure 4C).
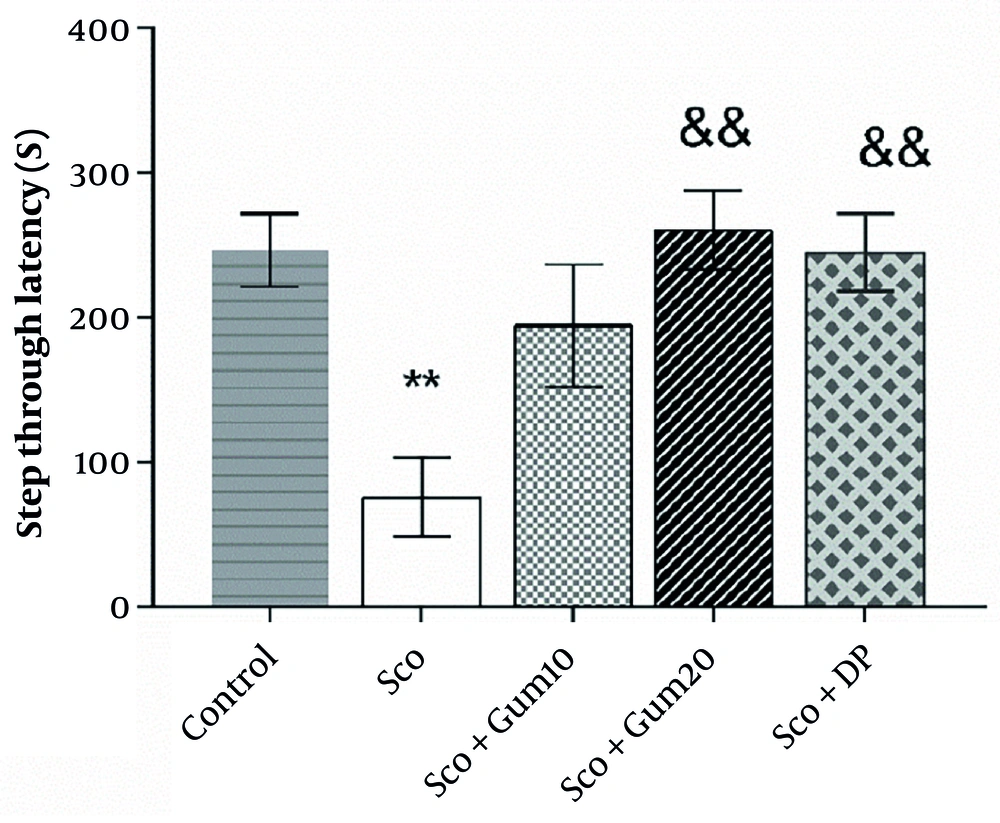
The effects of Gummosin injection in the model rats of memory impairment in passive avoidance test. Injection of both doses of Gummosin and donepezile resulted in recovery of STL time. All groups received an equal number of shocks. Data are expressed as Mean ± SEM. ** P < 0.01, compared with the control group. && P < 0.01 comparison with scopolamine group.
The effects of Gummosin injection in memory disorder model rats on memory and spatial learning in Morris Water Maze. Administration of Gummosin in Sco+Gum20 group rats improved spatial learning performance and significantly reduced escape latency during the first, second and third blocks. In the probe test, after removal of the platform from the target quadrant, cell transplantation in the groups receiving gummosin20 and Donepezile was accompanied by an increase in the percentage of time spent in the target quadrant. Swimming speed was the same in all study groups. Data are expressed as Mean ± SEM. * P < 0.05, ** P < 0.01, *** P < 0.001, comparison with the control group & P < 0.05 and && P < 0.01, compared with the scopolamine group.
3.1. Passive Avoidance Test Results
The value of step-through latency (STL) in the passive avoidance test is the time spent in the light area before the animal enters the dark area, with a decrease in STL considered an indicator of fear memory deficit. As shown in Figure 3, the STL time in the scopolamine group was 27.01 ± 75.86 s, which was significantly lower (P < 0.01) than the control group (246.6 ± 25.29 s). In this study, the number of shocks received by the animal is considered an indicator of fear memory. All groups received 1 - 3 shocks, and there were no differences between groups.
4. Discussion
The findings of this study demonstrate that scopolamine administration leads to deficits in memory, learning, and synaptic plasticity. These impairments were accompanied by altered gene expression and a reduction in hippocampal neuron numbers, further validating the scopolamine-induced Alzheimer’s disease model in alignment with previous research (11, 12). In contrast, treatment with gummosin at a dose of 20 mg/kg (scopolamine + gummosin 20 group) resulted in a significant increase in the time spent in the central area during behavioral testing, suggesting anxiolytic effects. A moderate improvement was also observed in the scopolamine + gummosin 10 group, though the effects were less pronounced, indicating that the higher dose may be more effective.
Passive avoidance testing revealed substantial improvement in fear memory for both the scopolamine + gummosin 20 and Sco + DP groups. The scopolamine + gummosin 10 group showed relative enhancement, albeit less significant. Similarly, in the Morris water maze, animals in the scopolamine + gummosin 20 and Sco + DP groups exhibited markedly improved memory and learning performance. Enhanced long-term potentiation (LTP) was recorded in both scopolamine + gummosin 20 and Sco + DP groups, further supporting the neuroprotective role of these treatments. The scopolamine + gummosin 10 group demonstrated limited effects, with no statistically significant difference compared to either the scopolamine or control groups.
Enhancement of LTP may result from functional and structural modifications in presynaptic, postsynaptic, or both types of neurons. To explore the underlying molecular mechanisms, gene expression levels were assessed via reverse transcription polymerase chain reaction (RT-PCR). The study revealed a significant reduction in CREB expression in the scopolamine-treated group. However, administration of donepezil and both doses of gummosin led to a partial restoration of CREB levels, correlating with improvements in synaptic plasticity and memory impairments. CREB is known to play a key role in synaptic plasticity, neuronal survival, neurogenesis, and cognitive function within the hippocampus and cortex (7). Additionally, glutamatergic transmission, particularly through NMDA receptor signaling, is critically involved in learning and memory processes (9).
In Alzheimer’s disease models, NR2B expression tends to increase (11). Donepezil treatment significantly elevated NR2A expression compared to scopolamine, while gummosin 10 and gummosin 20 also induced moderate increases in NR2A expression. In our experiment, scopolamine elevated NR2B levels, yet treatment with donepezil and gummosin 20 successfully restored NR2B expression to baseline levels. Gummosin 10 exhibited a milder recovery effect. Given CREB’s role in upregulating genes associated with neuronal survival and its protective function against apoptosis, and recognizing that NR2B is linked to cell death and suppresses NR2A activity, antagonizing NR2B receptors has emerged as a potential therapeutic approach for neurodegenerative conditions such as ischemia and dementia.
Taken together, the data suggest that gummosin, particularly at higher doses, may exert neuroprotective effects via dose-dependent activation of the NR2A/CREB signaling pathway, thereby promoting neuronal viability and enhancing cognitive functions such as memory and learning (12). Given that gummosin significantly reduced NR2B subunit expression in our study, it is plausible that this compound also promotes endogenous neurogenesis, thereby enhancing memory and synaptic plasticity.
Stereological analysis revealed that scopolamine administration induced notable structural alterations in the CA1 and CA3 regions of the hippocampus, findings consistent with earlier research linking scopolamine exposure to hippocampal disruption and corresponding behavioral impairments (2). Additionally, the present study demonstrated that donepezil effectively preserved neuronal density in the CA1 region of scopolamine-treated rats. Gummosin at a 20 mg/kg dosage yielded partial recovery in the CA1 region, exhibiting no significant difference from either the control or disease groups. However, gummosin treatment failed to reverse structural damage in the CA3 region.
Overall, our findings suggest that gummosin may facilitate functional recovery in a memory impairment model through a distinct mechanism from donepezil, primarily via enhancement of postsynaptic neuron function and activation of the NR2A/CREB signaling pathway, contributing to increased neuronal survival and improved learning and memory (4, 6).
4.1. Conclusions
This study represents the first report on the effects of gummosin administration in ameliorating scopolamine-induced memory impairment. Behavioral and electrophysiological assessments revealed that gummosin enhanced memory and learning in a dose-dependent manner. These improvements were associated with upregulation of NR2A and CREB gene expression and downregulation of NR2B expression. Specifically, gummosin 20 produced statistically significant changes, while gummosin10 elicited moderate effects, indicating that gummosin may promote neuronal survival and cognitive recovery through activation of the NR2A/CREB signaling pathway.
Despite these promising findings, further investigations are warranted to elucidate gummosin’s optimal therapeutic dosage and to explore its broader biological effects, including antioxidant properties, trophic support, immunomodulatory capacity, impact on synaptic plasticity, and efficacy in functional recovery in various animal models of neurodegenerative disorders. Such research will be essential before advancing toward clinical studies in humans.