Introduction
Listeria monocytogenes is an important foodborne pathogen. It plays a significant role in food safety control due to its wide distribution in nature.
The genus listeria contains 6 species of low G+C content gram-positive bacteria closely related to the genus bacillus [1]. The species are L. monocytogenes, L. ivanovii, L. innocua, L. seeligeri, L. welshimeri and L. grayi. Studies show that L. monocytogenes strains from food and food-environments are susceptible to the antibiotics commonly used in veterinary and human listeriosis treatment.
The treatment of choice for listeriosis remains the administration of ampicillin or penicillin G combined with an aminoglycoside, classically gentamicin. The association of trimethoprim with a sulfonamide, such as sulfamethoxazole in co-trimoxazole, is considered to be a second-choice therapy. Considering that L. monocytogenes is slowly becoming drug resistant, a continued surveillance of emerging drug resistance of this pathogen is important to ensure effective treatment of human listeriosis.
Antibiotics to which some L. monocytogenes are resistant include tetracycline, gentamicin, penicillin, ampicillin, streptomycin, erythromycin, kanamycin, sulphonamide, trimethoprim-sulfamethoxazole (co-trimoxazole) and rifampicin [2-4]. Studies about stress responses are very important in biological and medicine [5]. Microbial population encounter different environmental stresses and usually adaptation to these stresses causes extended tolerance to multiple other lethal stressors. This phenomenon is defined as stress hardening, which refers to the increased resistance to lethal factors after adaptation. A study by Lou and Yousef showed that treating L. monocytogenes with sub-lethal stresses increased resistance to lethal doses of the same stresses [6]. Mauro et al. reported that L. monocytogenes serotype 4b and 1/2c are become more resistant to sulphafurazole [4].
Although considerable progress has been made in understanding the L. monocytogenes stress response, but there are little reports concerning the effect of environmental stresses on increasing or decreasing antimicrobial susceptibility of L. monocytogenes to variety of antibiotics. After exposing to sublethal stresses, evaluating the increasing or decreasing cell survival of L. monocytogenes cells is significant in the biology of this pathogen. The aim of this work was evaluating the changes in drug resistance and cell survival of L. monocytogenes PTCC1297 (serotype 4a) to antibiotics after exposure to environmental stresses including acid, osmotic pressure, ethanol, oxidative and heat.
Results
In this study exponential phase of L. monocytogenes PTCC1297 (serotype 4a) cells was subjected to some sub-lethal environmental stresses such as ethanol (5% v/v), sodium chloride (7% w/v), acid (HCl, pH=5.0), hydrogen peroxide (600 ppm) and heat (45ºC) in order to evaluate changes in cell survival and susceptibilities to different antibiotics. As shown in the table 1, the most potent selected antibiotic is trimethoprim-sulfamethoxazole and the lowest activity was observed upon using rifampicin. Inductions of oxidative (600 ppm) and heat stresses at 45ºC lead to complete resistance to all antibiotics, but exposure to hydrochloric acid (pH=5.0), sodium chloride (7% w/v) and ethanol (5% v/v) stresses increased sensitivity to the antibiotics (p<0.05).
The minimum inhibitory concentration ranges (µg/mL) of L. monocytogenes PTCC1297 to selected antibiotics was shown in table 2. According to the obtained results, sensitivity to all antibiotics was in the approximate MIC breakpoints suggested by (CLSI) except the heat (45ºC) stress. Breakpoints for the used antibiotics are: penicillin (≤1.5), ampicillin (<1.5), tetracycline (≤4.0), chloram-phenicol (≤12.5), gentamycin (≤6.0), rifampicin (≤0.5), trimethoprim-sulfamethoxazole (≤0.5/9.5).
MBCs for penicillin, ampicillin, tetracycline, chloramphenicol, gentamycin, rifampicin and trimethoprim-sulfamethoxazole were determined. The MBCs end points for penicillin, ampicillin and tetracycline were about three-fold dilution of MIC. But the MBCs for chloramphenicol, gentamycin, rifampicin and trimethoprim-sulfamethoxazole were about two-fold dilution above the MICs.
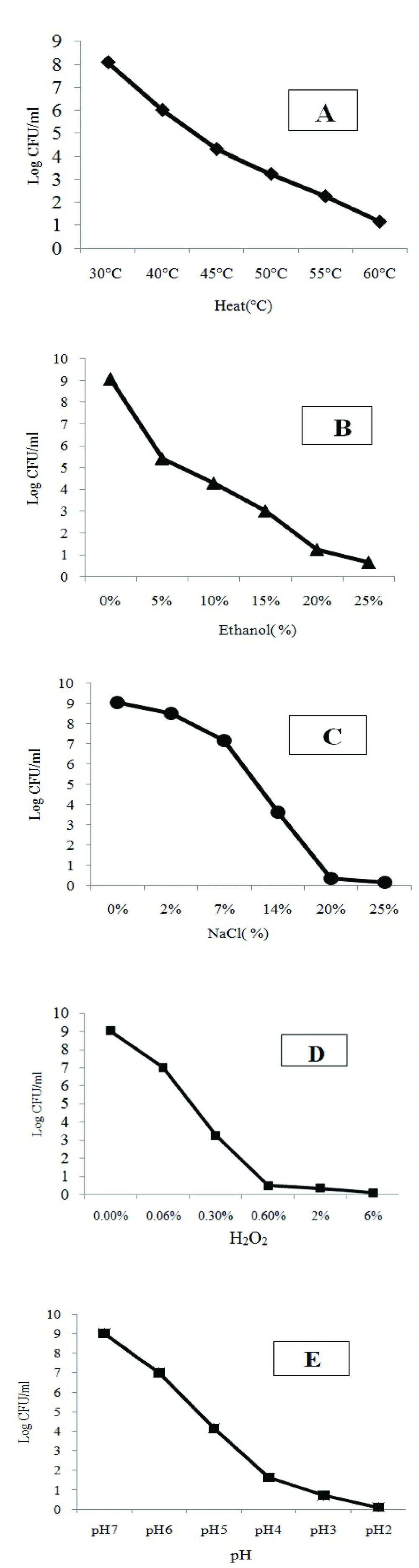
Figure 1 shows viability of L. monocytogenes PTCC1297 (serotype 4a) stressed cells exposed to different conditions and results are as follows:
Response to different temperature: L. monocytogenes stressed cells were cultured separately and exposed to 6 temperature conditions, (I) 30ºC, (II) 40ºC, (III) 45ºC, (IV) 50ºC, (V) 55ºC and (VI) 60ºC. The results showed that upon increasing the temperature viable cell decreased especially at 60ºC which is considered as lethal condition (Fig. 1A).
Response to ethanol and NaCl: The viability and resistance of stressed L. monocytogenes cells were increased to lethal doses of ethanol (14% v/v) and NaCl (20% w/v) concentrations as shown in (Fig. 1B, 1C) (p<0.05).
Response to hydrogen peroxide: Stressed L. monocytogenes cells treated with hydrogen peroxide (H2O2) at concentration of (600 ppm) showed increased resistance to antibiotics (p<0.05), but the viability decreased upon increasing hydrogen peroxide (H2O2) as shown (Fig. 1D) no growth occurred at 2.0%, 6.0% concentrations of hydrogen peroxide.
Response to acid conditions: Stressed L. monocytogenes cells were separately subjected to 5 acid conditions, (I) pH=2.0, (II) pH=3.0, (III) pH=4.0, (IV) pH=5.0, (V) pH=6.0. These treatments show that upon exposing to sub-lethal stress resistance of L. monocytogenes increased to high acid conditions (pH=3.0). L. monocytogenes growth was stopped in pH=2.0 (Fig. 1E).
| Sub-lethal stresses | Hydrochloric acid (pH=5) (mean±SD) | Sodium chloride (7% w/v) (mean±SD) | Ethanol (5% v/v) (mean±SD) | |
|---|---|---|---|---|
| Antibiotics | ||||
| Trimethoprim-sulfamethoxazole | 32-35 | 35-39±2.1 | 35-38±1.6 | 33-36±0.9 |
| Tetracycline | 21-26±1.3 | 28-32±1.3 | 34-36±2.4 | 27-30±1.3 |
| Chloramphenicol | 22-25±0.9 | 17-30±1.5 | 28-33±2.1 | 26-29±2.4 |
| Penicillin | 20-23±1.1 | 25-28±0.9 | 33-37±1.9 | 29-34±0.8 |
| Ampicillin | 16-18±1.6 | 19-23±1.2 | 25-28±2.7 | 23-26±2.2 |
| Gentamycin | 14-17±0.6 | 16-20±1.8 | 21-23±1.4 | 18-22±0.9 |
| Rifampicin | 9-11±0.5 | 11-14±0.9 | 10-13±1.2 | 7-11±1.9 |
Susceptibility of L. monocytogenes PTCC1297 to selected antibiotics before and after induction of stresses
| Sub-lethal stresses | Hydrochloric acid (pH=5) (mean±SD) | Sodium chloride (7% w/v) (mean±SD) | Ethanol (5% v/v) (mean±SD) | Hydrogen peroxide (600 ppm) (mean±SD) | Heat (45°C) (mean±SD) | |
|---|---|---|---|---|---|---|
| Antibiotics | ||||||
| Trimethoprim-sulfamethoxazole | 0.016-0.03±0.02 | 0.016-0.03±0.03 | 0.016-0.02±0.04 | 0.06-0.12±0.05 | 0.12-0.25±0.05 | 0.12-0.25±0.08 |
| Tetracycline | 0.12-0.5±0.03 | 0.12-0.25±0.06 | 0.06-0.25±0.05 | 0.12-0.25±0.03 | 0.25-0.5±0.05 | 0.5-1±0.03 |
| Chloramphenicol | 0.12-0.5±0.06 | 0.12-0.25±0.08 | 0.06-0.25±0.02 | 0.12-0.25±0.06 | 0.25-0.5±0.09 | 0.5-1±0.06 |
| Penicillin | 0.25-1.0±0.07 | 0.5-1.0±0.12 | 0.125-0.5±0.07 | 0.25-.05±0.05 | 0.5-1±0.03 | 1.0-2.0±0.07 |
| Ampicillin | 1.0-2.0±0.12 | 1.0-2.0±0.03 | 0.5-1.0±0.09 | 0.5-1.0±0.04 | 1.25-2±0.07 | 2.0-4.0±1.3 |
| Gentamycin | 1.0-2.0±0.16 | 0.5-1.25±0.16 | 0.5-1.25±0.02 | 0.5-1.25±0.07 | 1.25-2±0.1 | 2.0-4.0±1.2 |
| Rifampicin | 2.0-4.0±0.15 | 1.0-2.0±0.08 | 1.0-2.0±0.06 | 2.0-4.0±0.08 | 4.0-6.0±1.2 | 4.0-8.0±1.5 |
Minimum inhibitory concentration ranges (µg/mL) of L. monocytogenes PTCC1297 to selected antibiotics after induction of stresses
Viability curves of L. monocytogenes PTCC1297 exposed to different degrees of stresses (A) Heat, (B) Ethanol, (C) NaCl, (D) H2O2, (E) HCl incubation was performed in 250 mL-Erlenmeyer flasks containing 50 mL of listeria enrichment broth on a rotary shaker 150 rpm at 30°C for 24. Survivor plots (log 10 CFU/mL) were determined for each range of environmental conditions on listeria selective oxford agar
Discussion
In this study, exposure to hydrochloric acid (pH=5.0), sodium chloride (7% w/v) and ethanol (5% v/v) stresses increased sensitivity of L. monocytogenes to all selected antibiotics. It seems that acidification leads to proton and anion influx into the cytoplasm and disrupts metabolic functions as well as inducing damage to proteins, nucleic acids, and cell membranes, molecular processes and cellular components such as membranes, proteins, nucleic acids, and enzymes. The main reason for increasing the sensitivity to antibiotics is cellular damages caused by sub-lethal factors. Upon treating the cells to heat and H2O2 cell survival and resistance to antibiotics increased.
L. monocytogenes is an important public health problem. Also, as a foodborne pathogen, L. monocytogenes primarily targets immune compromised individuals, including the elderly, pregnant women and newborns, leading to listeriosis, a disease that, although less frequent in occurrence (2.4 cases/million), is associated with relatively high (averaging 20-30%) mortality rates [7].
L. monocytogenes is very important in food safety control due to its wide distribution in nature and its capacity to survive and grow on the food products in spite of exposure to stressful conditions associated with food processing and preparation. However, a phenomenon called stress hardening, which refers to the increased resistance to lethal doses of stress after exposing to sub-lethal environmental stresses may interfere with food preservation and safety. It is presumed that L. monocytogenes cells depend on various stress-sensing mechanisms [6].
Also, from the point of clinical view, for treatment of L. monocytogenes infections, ampicillin or penicillin is the drug of choice and synergistic combination of β-lactam with gentamicin is sometimes used in immune compromised and neutropenic patients. Microbiological studies have shown that these drugs are not bactericidal against L. monocytogenes [8]. Charpentier and Courvalin reported resistance of L. monocytogenes to different antibiotics such as tetracycline, gentamicin, penicillin, ampicillin, streptomycin, erythromycin, kanamycin, sulphonamide, trimethoprim, and rifampicin [3]. So, finding new ways to reduce resistance to different antibiotics is important in food industry and clinical therapy. Oxidative stress is deleterious to various. The responses of L. monocytogenes to sub-lethal dose of ethanol (5% v/v), acid (HCl, pH=4.5-5.0), H2O2 (500 ppm) were studied by Lou and Yousef [6]. They found that treating L. monocytogenes with sub-lethal doses of environmental stresses increases resistance of this strain to lethal effects of the stresses. Pre-incubation of L. monocytogenes at 3 acid conditions (I) pH=5.0, (II) pH=4.5, and (III) pH=5.0 significantly increased resistance of the pathogen to the lethal acid condition at pH=3.5. Also, adaptation to 5% ethanol or 500 ppm of H2O2 provided the greatest protection against H2O2. Protection against lethal levels of hydrogen peroxide may be partly explained by the induction of a sigma factor (σS, or KatF), which accounts for the general resistance to environmental stresses in microbial cells [9-11]. Oxidative stresses are under control of PerR, OhrR and σB¬factors [12, 13].
The presence PerR and Fur regulons during oxidative stress indicated a response to damage and/or repair proteins and DNA [14]. Our results showed that using H2O2 at 600 ppm leads to complete resistance to all selected antibiotics which may correlate to PerR and Fur regulons function, in contrast cell survival decreased in higher concentrations of H2O2.
We heated shocked L. monocytogenes cells by keeping it at 45ºC for 2 h and the cell survival and resistance to antibiotics increased to lethal doses of ethanol (14% v/v) and NaCl (20% w/v) concentrations, which is in consistent to the results obtained by Mead et al. [7]. They reported heat shocking significantly increased the resistance of L. monocytogenes to (25% w/v) NaCl. There are reports implying that exposing to ethanol stresses induce stress proteins with a profile similar to that of stress proteins induced by heat shock [15, 16]. So, we can conclude that stress responses to ethanol and heat shock act in similar ways in L. monocytogenes. Bergholz et al. [17] assessed genome-wide changes in the L. monocytogenes H7858 transcriptome during short-term and long-term adaptation to salt stress at 7°C and 37ºC to understand the impact of temperature on the responses of L. monocytogenes which uses to adapt to osmotic stress. They found that at both temperatures, the short-term response to salt stress increased transcript levels of sigB and sigB-regulated genes, as well as mrpABCDEFG, encoding a sodium/proton antiporter. This antiporter was found to play a role in adaptation to salt stress at both temperatures.
A study by Morvan et al. [18] showed that the prevalence of drug resistance strains of L. monocytogenes was estimated at 1.27% amongst 4816 isolates from human samples. An explanation for acid resistance is adaptive response of L. monocytogenes to weak or strong acid food preservatives includes an increase in the total lipid Tm (decreased membrane fluidity), decreasing the ability of the weak acid preservatives to pass through the membrane and to act into the microbial cell, and thus conferring protection. Furthermore, decreased membrane fluidity acts as strong defense mechanism in some conditions (in the cases of hydrochloric or acetic acid) or as mild defense mechanism (in the cases of benzoic or lactic acid) [19].
Altuntas et al. [20] reported that some L. monocytogenes strains were resistant to streptomycin and fosfomycin antibiotics. Also some studies showed that treating L. monocytogenes to sublethal concentrations of disinfectant such as benzalkonium chloride and triclosan reduced susceptibility to ciprofloxacin, gentamicin [21, 22].