Introduction
N-nitroso compounds (NOCs) are one of the important groups of carcinogens frequently present in human environment and food chain. Nitrosamines are formed endogenously from nitrate and nitrite under certain conditions such as the strong acidic pH of the stomach [1, 2]. Both environmental and food born N-nitrosamines pose a health risk for human and animals. Diethylnitrosamine is experimentally used to induce liver carcinoma and study the mechanisms of cytotoxic injury [3]. Diethylnitrosamine is reported to undergo metabolic activation by cytochrome P450 enzymes to form reactive electrophones which cause oxidative stress leading to cytotoxicity, mutagencity and carcinogenicity [1]. DEN is reported to induce generation of free radicals leading to oxidative stress and cell injury through its metabolized end product [4]. Various plants have been tested and found to be effective against diethyl nitrosamine induced carcinogenesis and toxicity [5].
Caffeine (1, 3, 7-trimethylxanthine) is a purine alkaloid present in many popular beverages, including cocoa, tea and coffee [6]. Caffeine and other methylxanthines are used in clinical medicine as diuretics, analgesics and muscle relaxants; and they can aid in the treatment of brain disorders such as headaches and Parkinson’s disease [7, 8]. The effect of caffeine on biological system has been examined and the results differ according to the dose tissue and duration of treatment. Some of the effects of caffeine may favor the production of free radicals and lead to a subsequent increase of lipid peroxidation by increasing oxidative stress [9, 10]. More recent observations suggest that it can also act as an antioxidant. The suggestions are largely based on chemical studies showing it to be able to scavenge reactive oxygen species (ROS), particularly the hydroxyl radical (OH), known to be generated in the body by irradiation with various electromagnetic frequencies such as exposure to UV, as well as by many ambient physiologic reactions involving oxygen utilization [11, 12].
In light of these observations, it was decided to evaluate the efficacy of caffeine, as an antioxidant against diethylnitrosamine induced renal damage.
Materials and Methods
Preparation of Chemical: Diethylnitrosamine (Sigma Aldrich, USA), and caffeine were obtained from sigma, USA.
Animals and treatments: For this experimental study, 40 female albino Wistar rats weighting 180±5 g were kept in the laboratory under constant conditions of temperature (24±2ºC) for at least 1 week before and through the experimental work, being maintained on a standard diet and water were available ad libitum. The animals were maintained in accordance with the guidelines prescribed by the faculty of sciences and the study was approved by the Animal Ethics Committee of the University of Shahid Charmin University, Iran. The experimental rats were divided into 4 groups:
Group I (control): Animals were fed on the standard diet and were served as control group. Group II (DEN): Rats were injected intraperitonealy with single dose of DEN 200 mg /kg [13, 14]. Group III (Caff): Animals were i.p. given 100 mg/kg caffeine, daily for 4 weeks. Group IV (DEN+Caff): Rats were injected with DEN followed by i.p. caffeine, daily for 4 weeks.
Biochemical assays: For biochemical study sera were obtained by centrifugation of the blood samples and stored at 20ºC until assayed for the biochemical parameters. The level of creatinine and BUN was assayed in serum were determined using commercially available kits (Pars azmoon Tehran, Iran).
Statistical analysis: Results are expressed as the mean ±standard error of mean (SEM). Within group comparisons were performed by the analysis of variance using ANOVA test. Significant difference between control and experimental groups was assessed by student’s t-test using SPSS-11. A probability level of less than 5 (p<0.05) was considered significant.
Results
Table 1 and table 2 show effect of single, daily 100 mg/kg bodyweight of i.p. caffeine on the average weight in DEN (200 mg/kg) nephrotoxic rats treated for 30 days. As indicated in the table, repeated i.p. injection with DEN induced significant progressive weight loss in the treated rats. However, weight loss was significantly enhanced by caffeine treatment in dose related fashion and the other hand, kidney weight also changes significantly by DEN treatment while recovery was seen by caffeine.
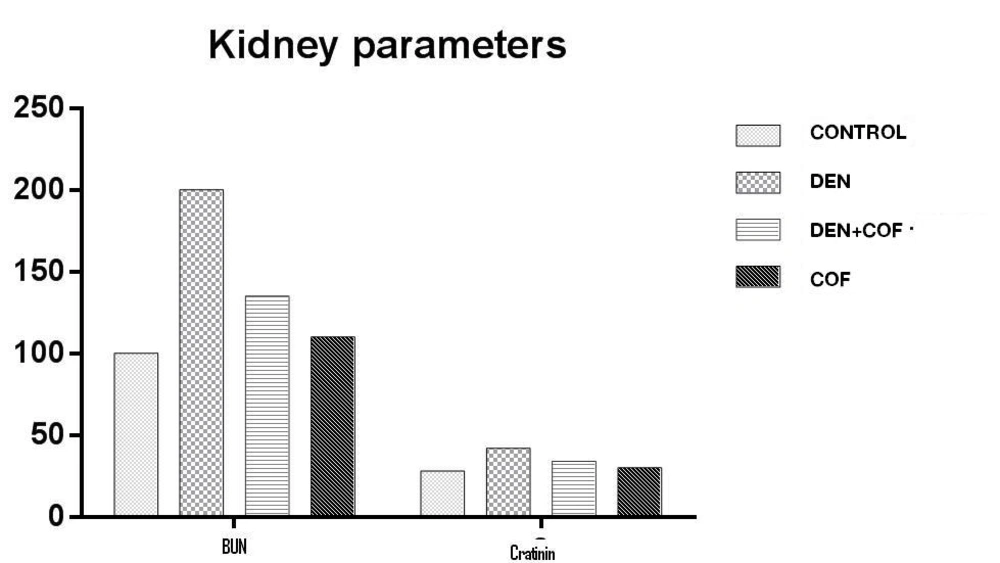
Figure 1 shows the status of serum creatinine and BUN in control and experimental animals. Diethylnitrosamine (group II) induced renal toxic is shown by a 2 fold increase in the level of creatinine and BUN in the serum of rats as compared to controls (group I). This increased level of creatinine and BUN in serum due to diethylnitrosamine challenge was significantly decreased on post treatment with caffeine (group IV) for 4 weeks.
| Group | Kidney wet weight (g) (Mean±SEM) |
|---|---|
| 1.6 ±0.05 | |
| 1.2±0.03 | |
| 1.7±0.13 | |
| 1.3±0.55 |
Average kidney weight changes in the caffeine group
Discussion
In the present study, serum levels of BUN and creatinine were significantly higher in diethylnitrosamine treated control and in experimental group in comparison to those of baseline control group. Again, significantly lower levels of these parameters were observed in experimental group when compared to those of N-diethylnitrosamine treated control group.
The kidney is an essential excretory organ of our body, plays a dominant role in homeostasis by excreting the metabolic waste products and excess necessary substances. Metabolites of the drugs that are excreted from kidney may also cause cellular damage leading to kidney dysfunction. Several xenobiotic substances exert their toxic effects by one or more common pathogenic mechanism that can produce nephrotoxicity [15]. Kidney disease is one of the commonest causes of hospitalization in most of the countries. Increase in the levels of blood urea and creatinine is the principal diagnostic criteria of renal failure. Severe and progressive uremia may result in death [16].
Diethylnitrosamine, one of the most important environmental carcinogen, has been suggested to cause the generation of ROS resulting in oxidative stress and cellular injury [17]. Diethylnitrosamine metabolized by cytochrome p450 generates a highly reactive free radical, and initiates lipid peroxidation of the cell membrane of the endoplasmic reticulum and causes a chain reaction. These reactive oxygen species can cause oxidative damage in DNA, proteins and lipids [1, 18].
The present study documents the antioxidant of the caffeine against kidney injury induced by DEN in rats. In this study, diethylnitrosamine administration to rats lead to a marked elevation in the levels of serum BUN and creatinine which is indicative of kidney damage, as previously reported [13, 19]. A single intraperitoneal dose of N-diethylnitrosamine (200 mg/kg body weight) increases microtonal lipid peroxidation and the activity of xanthenes oxidize and decreases the activities of renal antioxidant enzymes via, catalase, glutathione peroxidase, glutathione reductase and glucose-6-phosphate dehydrogenase, phase II metabolizing enzymes such as glutathione-S-transferase and quinone reductase and causes depletion in the level of renal glutathione content. A sharp increase in BUN and serum creatinine has also been observed [19].
Treatment with caffeine significantly reduced the level of the above marker parameters in diethylnitrosamine treated rats. This indicates that caffeine tends to prevent kidney damage. Haze et al. reported that administration of rats with caffeine alleviated the deleterious effect of cisplatin on kidney. They added that caffeine as an agent for reducing a renal toxicity with three mechanism, antioxidant, diuretic activities and blocking organic action transporter [20].
Caffeine is rich in photochemical derivatives such as triterpenes, flavonoids or polyphones. Many studies reported that the preventive effects of caffeine are attributed to its antioxidant activity [21]. Huber et al. reported that kahweol and cafestol phenol diterpenes of caffeine inhibit lipid peroxidation [22]. Lee et al. reported protective effects of caffeine on hepatotoxicity induced by carbon tetrachloride (CCl4) [23]. In conclusion, the present results showed that caffeine alleviates the renal toxicity induced by DEN in albino rats. This effect of caffeine may be attributed to the anti oxidant activity of one or more of its constituents.