Introduction
Atherosclerotic disease is the most important cause of mortality and morbidity in the world. Cardiovascular disease is the epidemic at present and until 2020 will be remained the single most important disease in the world in the terms of mortality, morbidity, disability and economic loss until 2020 year. This chronic disease has an enormous impact on quality of life [1]. Traditional cardiovascular risk factors such as hypertension, diabetes, dyslipidemia, smoking, and positive family history do not fully explain variations in the incidence of coronary disease [2].
About half of cardiovascular risk factors have not been completely understood. Trace elements play important roles in the maintenance of the normal physiology of cells. Association of trace element status and cardiovascular disease may be directly, through an effect on the vascular endothelium, or indirectly through the role of them in the lipoprotein metabolism. Several studies have been demonstrated lower serum level of manganese in atherosclerotic patients but none of them showed the relationship between serum level of manganese and the severity of atherosclerosis [3]. On the other hand, in some studies the manganese content of the heart and aorta of atherosclerotic subjects is lower and plasma level is higher than in healthy controls [4, 5]. We conducted this study to investigate whether a relationship exists between serum levels of manganese and the severity of atherosclerosis, measured by Syntax score.
Materials and Methods
This is a cross sectional study, performed on patients hospitalized in Sari heart center, Mazandaran Medical University from February 2010 to July 2012. Written informed consent was obtained from all enrollees, according to the criteria of the Ethical Committee of Mazandaran University of Medical Sciences. The sample consisted in 334 patients with chronic stable angina that each of them had been admitted for diagnostic coronary angiography for typical indications, such as evaluation of stable exertional angina.
The patients who had history of infectious disease in the recent two months, collagen vascular disease, and recent cardiac events were indicated ineligible. After coronary angiography all patients divided into 4 groups according to severity of coronary artery disease with the Syntax score. All groups were matched in cardiovascular risk factors.
Coronary angiography was performed by the Judkins technique through the femoral artery access and the angiograms evaluated by 2 cardiologists who were blinded to the study plan. The Syntax scoring system was used to determine the severity of coronary artery disease. Coronary artery disease was defined as >50% luminal diameter stenosis of the major epicardial coronary artery and categorized to mild for 1-22 scores, moderate for 23-32 and severe for 33and above it. The score is then multiplied by a factor representing the importance of the lesion’s location in the coronary artery system. For the location scores, 2 points were given for the partial occlusion of left main and 5 points for the total occlusion; 3.5 points for proximal stenosis of left anterior descending (LAD), 2.5 or 1.5 points for left circumflex artery stenosis depended to left or right dominancy; 1 point for RCA (right coronary artery) stenosis when there was right dominancy and 0 point when left dominant. Then the number of lesion complexity added to it.
Demographic characteristics: Cardiovascular risk factors including age, sex, systolic and diastolic blood pressure, smoking status, dyslipidemia, diabetes, were assessed for each subject. According to the New Zealand guideline, dyslipidemia was defined as total cholesterol to HDL more than 4. Hypertension was defined as a systolic blood pressure above 140 mmHg, or diastolic blood pressure above 90 mmHg, or current use of antihypertensive medication. Diabetes was defined as a known history of diabetes mellitus (fasting blood glucose 126 mg/dL or GTT higher than 200 mg/dL or treatment with Insulin or oral hypoglycemic agents. Smoking habit was categorized as non-smoker or ex-smoker in recent 1 year.
Biochemical evaluation: Blood samples were collected after an overnight fasting, immediately before the coronary angiography was started. They were centrifuged at 3000 g for 10 min at ambient temperature. The serum obtained was separated and frozen at -80ºC until the time of analysis. To determine manganese concentrations in serum samples, standard manganese solutions (Sigma chemical, Merck; containing from 0.05 to 1 mg/mL manganese diluted by 10% (v/v) glycerol) were prepared. After the samples were de-frozen, 1.5 mL of serum sample for manganese assessment was isolated. Manganese serum levels were assayed by flame atomic absorption spectrophotometer on an A100 variant. Then; the concentrations were determined following preparation of calibration curves and evaluation of line equation.
Statistical Analyses: Data were analyzed by the SPSS-16 software. Baseline demographic and laboratory data are presented for continuous variables as mean±SD and for discrete variables as frequencies. Parametric and non parametric data analyzed with t-test and χ2 between normal and total atherosclerotic groups. The mean difference of Mn level between four groups was analyzed using one-way ANOVAs. p<0.05 was considered statistically significant. We adjusted the role of age and sex by using General Linear Mixed Model.
Results
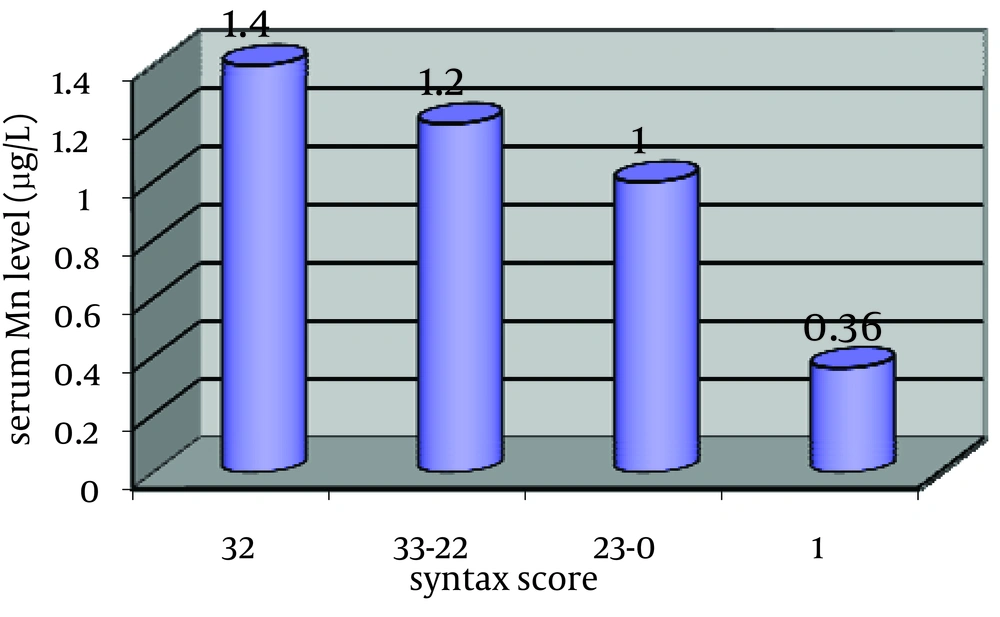
Comparison of demographic data between groups: Table 1 summarizes the demographic data for subjects. There was no significant difference in gender, age, smoking habits, occurrence of diabetes mellitus (DM) and hypertension (HT), serum TC/ HDL, and blood glucose levels between 4 groups. Serum manganese was 0.23±1.47µ g/L in normal coronary group. There were 0.24±1.28, 0.2±1.01 and 0.14±0.36µ g/L serum manganese in mild, moderate and sever CAD patients respectively.
The serum level of manganese in normal coronary group was significantly upper than the total atherosclerotic groups (p=0.001). The serum level of manganese was significantly decreased with severity of atherosclerosis and significantly lower in sever atherosclerotic patients (33 Syntax scores and above it) than mild and moderate CAD groups (p=0.001). Therefore, there were significant differences in serum level of manganese between normal coronary group and atherosclerotic patients and between CAD groups (Fig. 1).
| Characteristic | Normal coronary | Sever CAD | Moderate CAD | Mild CAD | p-Value |
|---|---|---|---|---|---|
| Blood sugar (mg/dL) | 60.09±129.42 | 59.70±134.29 | 79.52±141.57 | 60.28±131.35 | 0.18 |
| Systolic BP (mmHg) | 8.49±118.46 | 7.48±118.78 | 9.17±120.77 | 8.13±120.27 | 0.3 |
| Diastolic BP (mmHg) | 5.36±75.77 | 5.20±74.39 | 5.71±75.61 | 5.005±74.66 | 0.8 |
| TC/HDL | 1.27±4.61 | 5.14±1.49 | 1.18±4.56 | 1.17±4.56 | 0.7 |
| Age(yr) | 7.74±53.46 | 9.53±58.78 | 8.99±58.6 | 9.30±59.83 | 0.02 |
| Sex (male) N (%) | 19 (36.5) | 27(65.9) | 53(54.1) | 71(48.6) | 0.03 |
| Sex (female) N (%) | 33(63.5) | 14(34.1) | 45(45.9) | 75(51.4) | 0.03 |
Discussion
In this study, finding indicated that the serum level of manganese is lower in atherosclerotic patients and it decreases with severity of atherosclerosis. Coronary artery disease (CAD) is a leading cause of morbidity and mortality in developed countries and is emerging as an epidemic in developing countries [6].
There is strong evidence that oxidative free radicals have a role in the development of degenerative diseases including CAD. CAD is a leading cause of mortality, morbidity, and disability in Iranian population. It accounts for nearly 50% of all deaths per year in Iran caused for CAD [7]. Coronary disease has an enormous impact on quality of life [8]. Cardiovascular risk factors have not been completely understood [9, 10]. Coronary atherosclerosis has been associated with several risk factors including sex, age, dyslipidemia, diabetes mellitus, cigarette smoking and hypertension [11]. Several trace elements have also been implicated in the pathogenesis of CAD. Oxidative damage is thought to play an important role in atherosclerosis [12, 13]. Manganese is a trace element essential for cellular health, but toxic at higher levels [14].
Several studies have been demonstrated lower serum level of manganese in atherosclerotic patients but none of them showed the relationship between serum level of manganese and the severity of atherosclerosis. Kanabrocki et al. suggested that urine content of manganese is significantly higher in patients with coronary disease than in healthy subjects [15] but in our study we measured serum level of manganese that was significantly higher in normal group than CAD patients. In Volkov and Masironi studies, the manganese content of the heart and aorta of atherosclerotic subjects is lower and plasma level is higher than in healthy controls [4, 5]. In our study plasma level of manganese is higher than in healthy controls too.
In Cebi et al. study, the manganese concentrations was not significantly changed in patients with coronary artery disease [6] but in our study, CAD patients had significantly lower level of manganese than normal subjects and this association existed in all CAD groups. This difference probably due to little sample volume in Cebi et al. [6] study and it seems to be this matter affected their study accuracy. We aimed to determine the relationship between serum level of manganese and severity of the coronary atherosclerosis.
In conclusion, we have detected lower serum level of manganese in patients with atherosclerosis than the normal group; also we observed lower serum manganese concentrations to be associated with increased Syntax score and atherosclerosis severity. The present data suggest the protective role of manganese so that low levels of this element may play a role in atherosclerotic process. Enrolling the small number of patients to the study may be our study’s limitation and we hope that distributed study will be performed. Our study confirmed the basic relationship between serum manganese level and atherosclerosis and an association between manganese level and severity of atherosclerosis. Hence, further and more prospective studies are needed to confirm the relationship between this element level and the severity of atherosclerosis.