Introduction
There is a continuing search for bone substitutes to avoid or minimize the need for autogenous bone grafts. The use of bone grafts in the management of nonunion cases is well accepted. These grafts act as scaffolds, which provide the necessary biomechanical strength that is required to withstand the compressive forces involved during motion. They also promote the ingrowth of cells and other biological products, which eventually leads to the replacement of these grafts by bioactive tissues [1]. Autogenic and allogenic bone grafts are commonly used to treat these conditions. However, the limited availability of graft sites and the donor-site morbidity associated with the use of autologous bone grafts have been a major concern. In allogenic bone grafts, concerns over transmissible diseases and risk of contamination remain high, making its use less appealing for patients. Bone grafts are generally made from either biological material, for example, hydroxyapatite or synthetic materials, for example, calcium carbonate. Each of these grafts has unique advantages when used in patients; however, the common deficiency seen in these grafts appears to be in their ability to promote early bone incorporation, which, in turn, translates to late healing. The use of osteogenic promoters includes biological substances, cell therapies and mechanical induction [1]. These therapies are still novel and expensive with the exception of one product: PRP (platelet- rich plasma). PRP is not only easy to obtain and produce, but also safe [2, 3]. In many literatures, PRP has been used to improve bone healing in the fields of orthopaedic and maxillofacial surgeries; however, reports on the benefits of this material have not been conclusive as there have been contradictory outcomes, which report both good and poor results [2-8]. Clinical studies data were influenced by variables, such as defect size, site and patient factors that were not standardized, whilst the few experimental studies carried out did not demonstrate clearly if the PRP used had comparable concentrations. A study to determine the true effectiveness of PRP in treating non- or delayed union is, therefore, necessary to justify its use in clinical practice. In this study, a standardised technique to treat a delayed union model was performed in phenotypically identical rabbits and assessed for healing using biomechanical, histological and radiological methods to illustrate the role of PRP in enhancing bone healing.
Results
No animal was died during operation or until the end of the experiment and all the animals completed the study without any complications.
Radiographic findings
Bone formation: There was 0-25% bone formation in some rabbits in the control group, however 25-50% bone formation was observed in the defect of the animal of hPRP group on 14th postoperative day. The statistical tests supported significant difference on 14th postoperative day for bone formation (p=0.001).
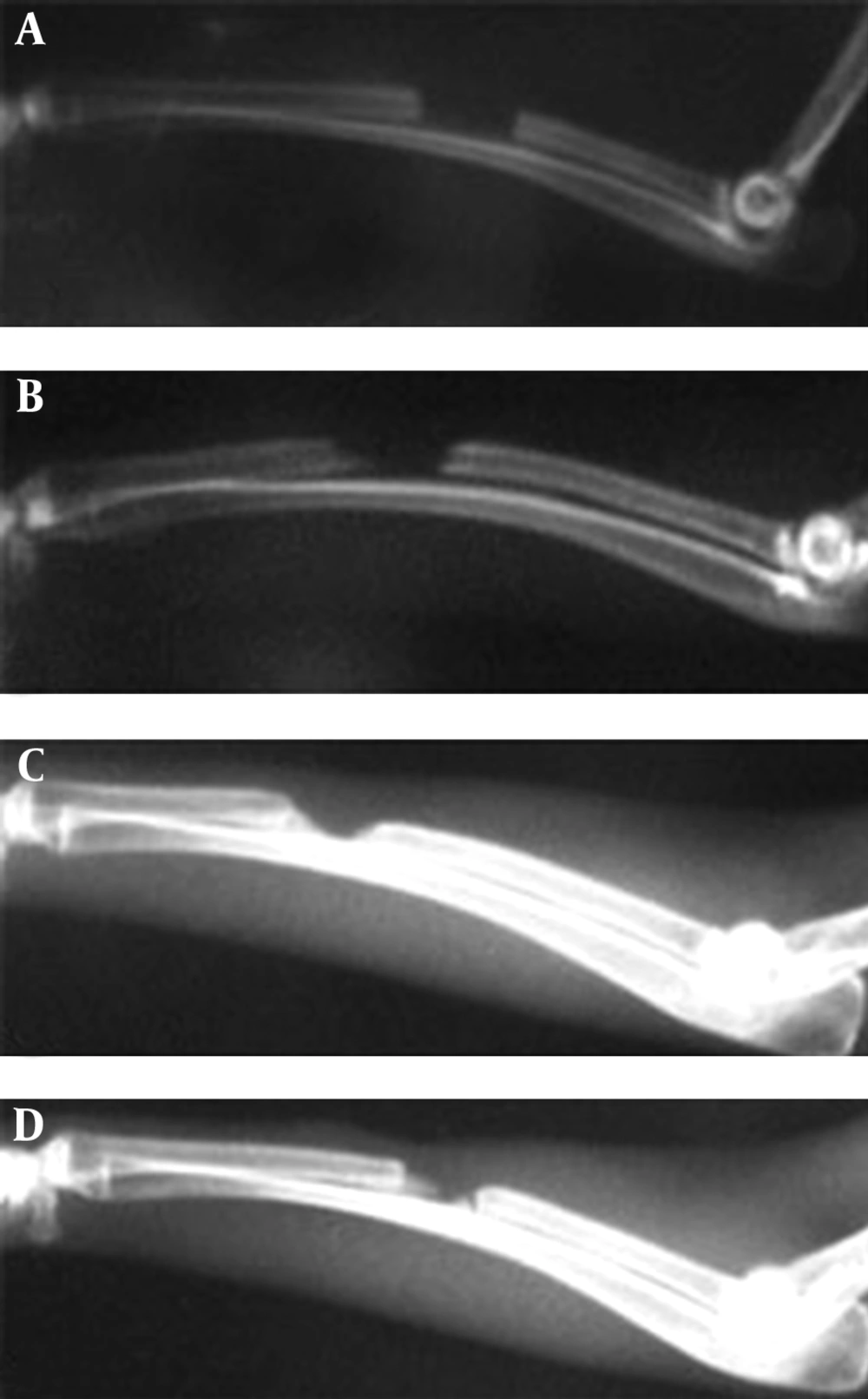
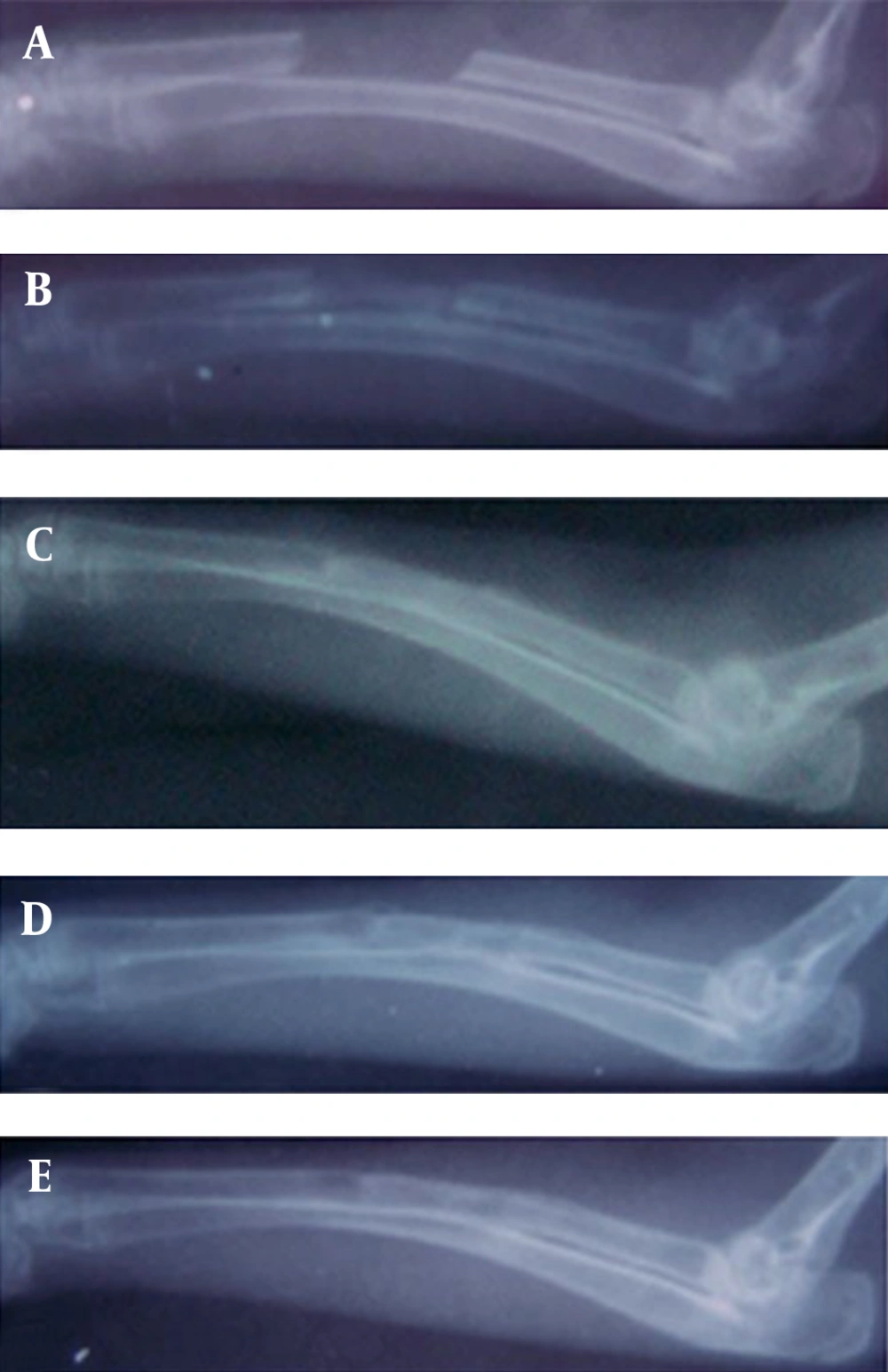
There was significantly more (50-75%) bone formation activity in the defects of the rabbits of hPRP group compared to those of the control group (0-25% bone formation) on 28th postoperative day (p=0.002). On 42nd postoperative day there was 75-100% bone formation in all rabbits in hPRP group while 25-75% bone formation was seen in the rabbits of the control group (p=0.002). There was 100% bone formation in the animals of the hPRP group and 50-75% bone formation in those of the control group on 56th post operative day (Table 1, Fig. 1 and 2).
Bone union: There was bone union in the rabbits of hPRP group and there was no evidence of union in the rabbits of the control group on 14th postoperative days. In addition, there was significant bone union in the rabbits of hPRP group compared to those of the control ones on 28th postoperative day. There was statistically significant difference for bone union at the 42nd and 56th post operative days in the radiological signs of bone union between the hPRP and control group (p<0.05) (Tables 2 and 3, Fig. 1 and 2).
Remodeling: Remodeling was not found in either group on 14th, 28th and 42nd postoperative days. On 56th postoperative day remodeling was observed in the rabbits of the treated group and statistical tests revealed significant difference between the 2 groups, and the operated area of the hPRP group showed a more advanced remodeling compared to those of the control one (Table 4, Fig. 1 and 2).
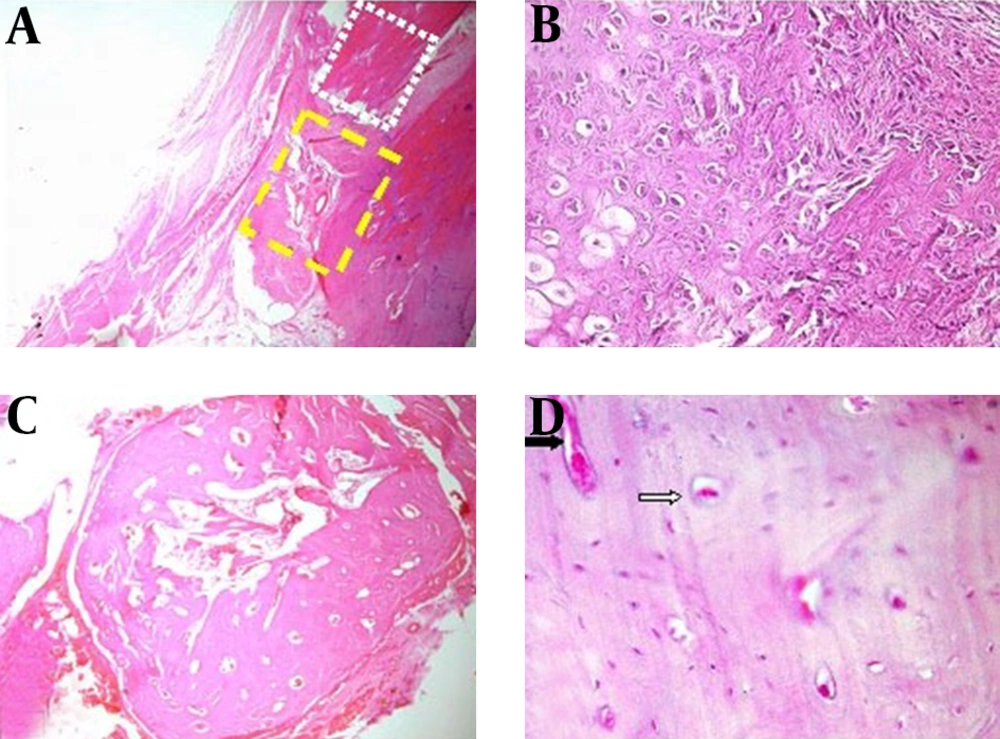
Histopathological findings: At histopathological level, the defects of the animals of the hPRP group showed more advanced repair criteria [med (min-max), 7 (6-7)] than those of the control group [med (min-max), 2 (1-5)] and statistical tests revealed significant difference between the two groups (p=0.002). In all of animals of hPRP group the defects were filled with remodeled bone tissue and histological union developed in any of these animals. Fibrous nonunions or fibrocartilages in the bone defects of the animals of the control group were dominant and the lesions of these animals showed poor re-vascularization. Bridging callus or histological union did not develop in any of these defects. These criteria lead to very slow repair process in the animals of the control group (Fig. 3).
Biomechanical properties: There was significant difference between the injured bone with normal bone of the control group in terms of ultimate strength (p=0.01) and stiffness (p=0.04) and the normal bones had superior ultimate strength and stiffness compared to their normal contralateral bones. In addition, the ultimate strength (p=0.03) and stiffness (p=0.02) of the treated limbs showed more advanced values in comparison with those of the control group (Table 5).
Photomicrograph of control group note to fibrous connective tissues in the defected area without bone marrow formation (yellow rectangle), note to old bone region (white rectangle) (A, H & E stain 4x). Note to extensive fibrocartilage tissue (B, H & E stain 40x). Photomicrograph of hPRP group, compact cortical bone and marrow formation was observed in grafted area (C, H & E stain 4x). Note to compact bone formation with several Haversian canal (white arrow) and Volkmann canal (black arrow) (D, H & E stain 40x)
| Med (min-max) | p-Value | ||
|---|---|---|---|
| Postoperative days | Control (N=6) | hPRP (N=6) | |
| 0 (0-1) | 1 (0-2) | 0.001 | |
| 1 (0-1) | 2 (1-3) | 0.002 | |
| 1 (0-3) | 3 (1-4) | 0.002 | |
| 2 (1-3) | 3 (2-4) | 0.001 | |
Significant p-Values are presented in bold face.
aKruskal-Wallis non-parametric ANOVA
bp= 0.002 (compared with control by Mann-Whitney U test)
cp= 0.007 (compared with control by Mann-Whitney U test)
dp= 0.002 (compared with control by Mann-Whitney U test)
ep= 0.001 (compared with control by Mann-Whitney U test)
| Med (min-max) | p-Value | ||
|---|---|---|---|
| Postoperative days | Control (N=6) | hPRP (N=6) | |
| 0 (0-0) | 0 (0-2) | 0.03 | |
| 1 (0-1) | 2 (0-2) | 0.008 | |
| 1 (0-1) | 2 (1-2) | 0.001 | |
| 1 (0-2) | 2(1-2) | 0.001 | |
Significant p-values are presented in bold face
aKruskal-Wallis non-parametric ANOVA
bp= 0.01 (compared with the control group by Mann-Whitney U test)
cp= 0.001 (compared with the control group by Mann-Whitney U test)
dp= 0.002 (compared with the control group by Mann-Whitney U test)
| Med (min-max) | p-Value | ||
|---|---|---|---|
| Postoperative days | Control (N=6) | hPRP (N=6) | |
| 0 (0-1) | 1 (0-1) | 0.004 | |
| 1 (0-1) | 2 (0-2) | 0.002 | |
| 2 (0-2) | 2 (0-2) | 0.005 | |
| 2 (0-2) | 2 (0-2) | 0.04 | |
Significant P-values are presented in bold face
aKruskal-Wallis non-parametric ANOVA
bp= 0.01 (compared with the control group by Mann-Whitney U test)
cp= 0.03 (compared with the control group by Mann-Whitney U test)
dp= 0.007 (compared with the control group by Mann-Whitney U test)
| Med (min-max) | p-Value | ||
|---|---|---|---|
| Postoperative days | Control (N=6) | hPRP (N=6) | |
| 0 (0-0) | 0 (0-0) | 1.000 | |
| 0 (0-0) | 0 (0-1) | 0.3 | |
| 0 (0-0) | 0 (0-1) | 0.03 | |
| 0 (0-1) | 1 (0-2) | 0.01 | |
Significant p-values are presented in bold face
aKruskal-Wallis non-parametric ANOVA
bp= 0.01 (compared with group II by Mann-Whitney U test)
| Mean±SEM | ||||
|---|---|---|---|---|
| Control (N=6) | hPRP (N=6) | |||
| Three point bending test criteria | Normal limb | Treated limb | Normal limb | Treated limb |
| 74.33±10.0 | 38.6±7.5 | 98.6±7.7 | 99.1±19.1 | |
| 3.64±0.7 | 2.18±0.3 | 6.08±0.77 | 6.28±0.69 | |
| 128.3±7.4 | 91.6±14.9 | 118.3±14.4 | 105.0±5.0 | |
| 7.9±0.5 | 8.4±0.6 | 8.52±0.4 | 8.1±0.1 | |
ap= 0.01 (normal limb compared with treated limb in control group by student-t test)
bp= 0.03 (compared with treated limb in control group by by student t-test)
cp= 0.02 (compared with treated limb in control group by by student t-test)
dp= 0.04 (normal limb compared with treated limb in control group by student t-test)
Discussion
This study was designed to provide an explanation for the existing confusion in the literature regarding the efficacy of the PRP application, and to give more insight into the effect of PRP on bone regeneration. To the authors’ knowledge this is one of the first studies, which presents new data on the bone regenerative properties of the human PRP as a xenogenic PRP on bone repair in rabbit model. Such defect in the radius in the rabbit model has previously been reported suitable because there is no need for internal or external fixation which influences the repair process [9]. The segmental defect was created on the middle portion of the radius as long as 10 mm to prevent spontaneous and rapid healing [9].
The clinical and experimental data in the literature regarding the osteogenic potential of PRP are controversial. The results of the present investigation confirm a number of clinical and experimental studies demonstrating a positive influence of PRP on bone regeneration [2, 4]. However, in human maxillofacial defects, neither autograft nor allograft or a mineral bone substitute material enhanced bone formation when augmented with PRP [17-19]. In a non-critical rabbit skull defect, PRP was not superior to the empty defect nor did PRP increased bone formation by autogenous bone [20].
The results of the present study indicate that hPRP stimulates a favorable reaction in the injured area of the long bones. The radiographic evaluation at two week post-injury showed that the bone gap in the hPRP group was healed before that of the control group and it was also already in the remodeling stage. While the defects of the control animals were still in the healing stage even at the end of eight weeks post-injury. This fact was corroborated by histopathologic and biomechanical data analysis, which showed that osteogenesis in the animals of hPRP group at 56 days post injury was stronger than those of the control group.
PRP contains several growth factors including isomers of PDGF, TGF-X 1, TGF-X 2, IGF-I, IGF-II and VEGF that all of them are promotors of bone regeneration. PDGF has been shown to be mitogenic for osteoblasts [21] and stimulates migration of the mesenchymal progenitor cells [22]. It is stated that in the bone defects of the animal models, PDGF induced callus formation [23]. TGF-X also has a stimulative effect on osteogenesis and inhibits bone resorption [24]. In addition, it has been reported that IGF-I and the angiogenic factor VEGF induced bone formation in rats [25] and in rabbits [26], respectively. These growth factors support bone regeneration primarily via chemotactic and mitogenic effects on preosteoblastic and osteoblastic cells. Due to this phenomenon, an enhanced bone formation criteria in the animals of the hPRP group compared to those of the control ones was observed. However, hPRP does not contain BMPs, the most potent osteoinductive proteins, which promote stem cell differentiation into the osteoblastic lineage and are the only growth factors known to induce ectopic bone formation [27]. Schlegel et al. and Thorwarth et al. got better results by administering higher doses of hPRP (6.5-fold compared to normal blood) than with lower platelet concentrations (4.1-fold) on bone regeneration in skull defects of minipigs [28, 29]. Other experimental studies found no correlation between the platelet concentration with improvement in bone repair [20, 30]. In the present study, high platelet concentrations (10.1-fold compared to normal blood) were effective and lead to superior and faster bone formation than those of the control group.
The biomechanical evaluation performed in the present study indicated initial failure at the interosseous membrane, suggesting a strong load sharing mechanism through this syndesmosis between the radius and the ulna. The syndesmosis was shown to have extensive calcification, accounting for a large fraction of the bone volume in the defect and possibly contributed to the bone in-growth into the defected area. This was supported both by histopathologic and radiographic evidences showing new bone growth in a cone-like fashion and from the direction of the interosseous membrane in the defects of hPRP group as well as in the defects with no treatments. Thus, separating the radius from the ulna for biomechanical testing may damage this tissue. It is also important to consider that the radius and ulna act as a unit in the physiological setting and it may be more biologically-relevant to evaluate them together [31].
Based on the radiological, histopathological and biomechanical findings of the present study, healing of the defects of the animals of the control group was not very efficient and the defect area was filled with fibrous tissues and rarely with cartilage instead of osseous tissue. Barnes et al. indicated that the chondrocytes derived from the mesenchymal progenitors proliferate and synthesize cartilaginous matrix until all the fibrous granulation tissue is replaced by cartilage. Where cartilage production is deficient, fibroblasts replace the region with generalized fibrous tissue. Discrete cartilaginous regions progressively grow and merge to produce a central fibrocartilaginous plug between the fractured fragments that splints the fracture [32].
Preparation of PRP from the animal blood is not a standardized procedure such as PRP preparation from human blood, therefore, it was not possible to purchase the rabbit PRP from the well known markets. Preparing the rabbit PRP from the same animals needed a sizeable volume of blood to be collected from each rabbit and the Ethics Committee laws did not permit to collect such amount of blood from rabbits and believed this will affect their healing potentials. In addition, the critical effective amounts of platelets in PRP for different animal species, levels of growth factors in different animal species and similarities or differences in their mechanisms of action with PRP of humans have still to be defined. Until then, the animal PRP preparations/studies should be interpreted carefully [33]. Production of xenoreactive antibodies against hPRP could not be excluded, which might have affected the results, however, in the current model, no histological signs of acute or chronic inflammatory response in hPRP xenograft was observed, although it may have been present earlier. As it is stated in the text one of the main reasons was that the properties of hPRP contents have been proved in many earlier studies, however, the rabbit’s PRP contents is still unknown to our knowledge. In overall, this study showed that hPRP has a high regenerative capacity in critical size bone defects in rabbit model after 8 weeks and it might be used to accelerate the bony defect healing in orthopedic or maxillofacial reconstructive surgery.