Introduction
Calcium is the most important mineral in human’s body. As well as a structural role in teeth and bones, it has important physiological functions such as muscle contraction, neural excitation, blood clotting, secretion, fertilization, enzyme activation, proliferation, gene expression and apoptosis [1, 2]. There are three forms of calcium in plasma: free calcium ion, binding to proteins such as albumin and binding to small anion molecules such as phosphate, bicarbonate, and citrate [3]. According to the importance of various physiological roles of calcium in human’s body, measuring serum level of calcium is a daily demand of physicians and referring of medical laboratories [4]. Usually some tests such as complete blood count (CBC) and measurement of plasma glucose requires plasma sample. The most common anticoagulant to obtain plasma is ethylene diamine tetra acetic acid (EDTA). Due to the lack of calcium assay on plasma samples containing EDTA, laboratories should provide serum samples for measurement of calcium. Conventional methods for measuring blood calcium include: Clark-Colip method which is the oldest one and involves complicated procedures such as the precipitation of calcium as the oxalate, separation of precipitated oxalate by filtration, release of oxalate ions in hot H2SO4 solution, and titration of the oxalate with potassium permanganate. This standard method is usually used to check the accuracy of the other methods [5], complexometry or the titration method of calcium with EDTA is a rapid and accurate one but recognition of endpoint of titration is difficult [6]. The various enzymatic assays have been reported but they have limitations due to their instability of the reactants [7]. Methods such as atomic absorption spectrometry (AAS) [8], ion selective electrodes (ISE) [9], ion chromatography [10], nuclear magnetic resonance (NMR) [11], high performance liquid chromatography (HPLC) [12], capillary electrophoresis [13] are analytical methods which are sensitive but they are sophisticated and their procedures include tedious and cumbersome extraction or preparation steps. In addition, the costs of these instruments are high [14]. Currently the most common method for measuring calcium levels in clinical laboratory is a spectrophotometric method using Ortho cresolphthalein complexone (o-CPC) [15, 16]. The most important limitation of o-CPC is its inability in measuring calcium in EDTA plasma because EDTA with calcium ion forms a stable complex. Thus, for measuring calcium by this reagent, it is strongly emphasized not to use EDTA plasma. Since it has not been reported any routine laboratory method for measuring of calcium levels in EDTA plasma samples, the need to conduct such a study was found. In this study a new patented method has been presented, according which we are able to measure calcium in EDTA treated plasma by o-CPC.
Materials and Methods
In this experimental study about 4 mL blood was taken from 40 apparently healthy volunteers. Each sample was divided into two aliquots (2 mL for preparation of serum and 2 mL for preparation of plasma). For plasma preparation, tubes containing EDTA was used. Samples were collected in tubes and stored in microtubes without any contamination of calcium. For preventing surface evaporation, microtubes caps were completely sealed. Until testing, the samples were placed at 2-8ºC and for longer periods they were kept at -20°C. All chemicals were purchased from Merck, Germany.
For calcium determination at first 10 µL of plasma samples, standard as a calibrator and distilled water as a blank, were added to the wells of a 96 wells microplate. Calcium standard solution (10.5 mg/dL) was purchased from Pars Azmoun Co., Tehran, Iran. Solution of X was prepared. (X is a patented solution that can dissociate and release calcium from EDTA. It is patented on 29.04.2012 in State Organization for Registration of Deeds and Properties with registration number of 74, 804, and is the exclusive property for the owners). Then 10 µL of X solution was added to wells containing plasma and blank. For having the same volume, 10 µL double distilled water was added to the standard solution. For 15 min, microplate was placed on a microplate shaker (Stat Fax-2200, Awareness Technology, Inc., USA). Four parts of ethanolamine solution, 0.8 M/L, pH=10.7, and one part of solutions containing o-CPC, 0.06 mM, 8-hydroxyquinoline, 7 mM and hydrochloric acid, 20 mM, pH=1.1 for making a homogenous solution were mixed. Then 300 µL of the homogenous solution were added to the all wells containing plasma, standard and blank. Finally, after 5 min the absorbance was measured at 578 nm with a microplate reader (Sun Rise, TECAN A-5082, TECAN Groups Ltd., Salzburg, Austria).
The validity of the method has been evaluated as follows: Precision of the method was assessed by intra and inter-assay coefficient of variations percent for three samples with low, medium, and high calcium content. Replication number was eight in all steps. Sensitivity of the method was calculated based on average optical density of zero standards plus twice the standard deviation. To evaluate the accuracy based on parallelism test, a sample was serially diluted with double distilled water. Using the expected and measured data, the ratio percentage of parallelism tests was determined. Also, the recovery test was determined by adding 10 µL of standard solution into three samples with different concentrations. Then using the expected and measured data, the recovery percent was calculated. For comparing two methods, the obtained results of this new method in plasma samples were compared with o-CPC colorimetric method in the same serum samples and the correlation coefficient was calculated.
Results
After theoretical studies and pilot tests, X was chosen as a displacer of calcium ion from EDTA as a chelators. Proper concentration and volume of X and the required time for releasing of calcium from the EDTA-Ca complex were optimized. Table 1 contains the data of inter/intra assay. As the findings show, the precision of the method is acceptable.
| Run | Concentration | Replications No. | Mean±SD | CV% |
|---|---|---|---|---|
| Low | 8 | 7.98±0.20 | 2.57 | |
| Medium | 8 | 10.03±0.44 | 4.34 | |
| High | 8 | 11.64±0.48 | 4.10 | |
| Low | 8 | 8.92±0.52 | 5.83 | |
| Medium | 8 | 10.62±0.62 | 5.85 | |
| High | 8 | 12.16±0.65 | 5.36 |
Based on the average of zero standard absorption plus twice the standard deviation, the sensitivity was calculated 0.4 mg/dL
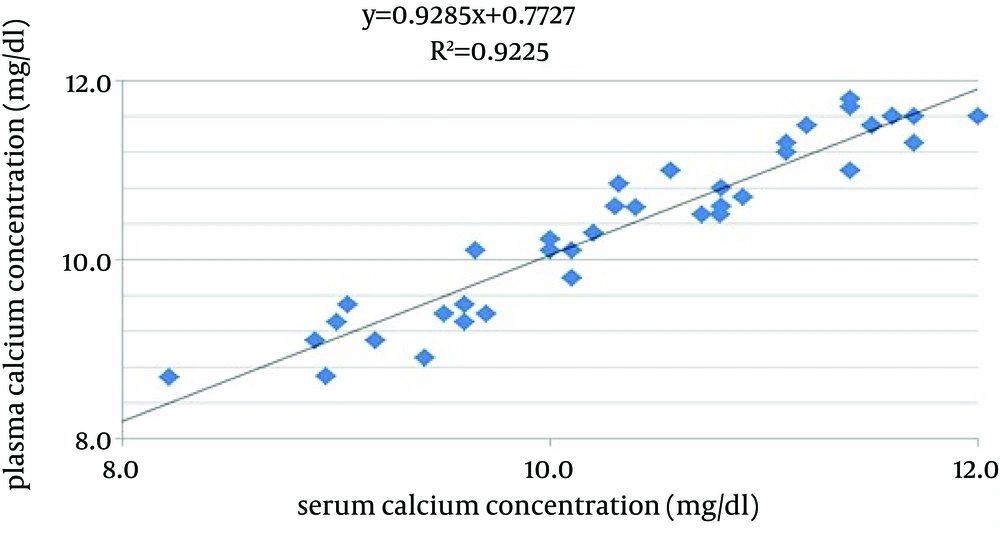
The results of parallelism and recovery tests were presented in tables 2 and 3. These tables showed that the method has an acceptable accuracy. Figure 1 contains the results of method comparison.
| Sample | Dilution | Expected | Measured | Ratio% |
|---|---|---|---|---|
| 1 | 7.38 | 7.38 | 100 | |
| 2 | 3.69 | 3.75 | 101.69 | |
| 4 | 1.84 | 1.58 | 85.42 | |
| 8 | 0.92 | 0.88 | 94.92 |
| Sample | Standard | Expected | Measured | Recovery% |
|---|---|---|---|---|
| 11.10 | 10.35 | 10.76 | 103.92 | |
| 11.10 | 11.28 | 11.29 | 100.07 | |
| 11.10 | 11.81 | 12.99 | 110.00 |
Discussion
According to data collected in present study, patented solution X, can dissociate and release calcium ions from EDTA chelator and provides the possibility of measuring calcium concentration in EDTA plasma. Since it has not been reported any routine assay for calcium determination in EDTA treated plasma, comparative evaluating of patented method is not possible. Therefore, the scientific basis of patented solution, efficiency and its advantages compared with other methods are discussed. In EDTA treated plasma, calcium is highly chelated by EDTA and is not exposed to o-CPC, because EDTA has a high tendency to form complex with calcium ion. The scientific basis of the novel method is formed using a displacer. Accordingly, if a displacer which has a greater tendency to EDTA is added to EDTA plasma, Ca is released by replacement. After the release of calcium ions, they can be determined by o-CPC reagent.
Despite the importance of calcium in various metabolic processes, there is not a method for calcium determination in plasma. Additionally many methods for calcium determination require analytical instruments which are not proper for clinical laboratories because of their high costs, high experienced personnel and low rate of measurement [17]. However, these methods have the high accuracy and are considered as the reference method [5-14]. So a simple and inexpensive method is needed.
Colorimetric method for calcium determination by o-CPC reagent due to its simplicity, sensitivity and efficiency, is common. But this method is limited to use of urine, serum and heparin plasma samples [15, 16]. Heparin is more expensive than the other anticoagulant reagents (e.g. EDTA) and its effect is temporary. Heparin does not stop clotting of blood and it just delays the process for 8 to 12 h [18]. Thus, the most commonly anticoagulant is EDTA. So according to o-CPC method, laboratory has to take serum and plasma, 2 kinds of samples from patients who need complete blood count (EDTA treated blood) and serum calcium. Due to the mentioned reasons, efforts was started for removing the limitation of o-CPC method and eventually leads to develop a new method for measuring calcium in EDTA treated sample which is patented on 29.04.2012 in State Organization for Registration of Deeds and Properties with registration number of 74804. Generally, solving the limitations of common method of calcium determination improves methodological parameters and expands the range of applied science in this field. Another advantage of this method is that calcium can be measured in plasma and serum simultaneously. Thus, the laboratory costs are reduced. Thirdly, this method is a simple method that can directly measure the calcium. Also microplate reading format increased speed of the assay time and shorten the total assay time.
The obtained results of this new method in plasma samples should have been compared with another method that determines the calcium levels in EDTA plasma sample, but as mentioned before the reported methods for calcium determination have been used serum sample not plasma. Since the difference between plasma and serum calcium is low and it is not possible to determine EDTA plasma calcium level, so they obtained results of the new method were compared with identical serum samples. Final conclusion of this study is the proposed method for measuring calcium levels in EDTA plasma has an acceptable sensitivity, precision and accuracy. According to this method, limitation of colorimetric method for EDTA plasma calcium determination by o-CPC reagent was solved. Since the most clinical laboratories use auto analyzer for biochemical analysis and measurements, it is suggests to study the possibility of adjust this new method on common automated instruments.