Introduction
Leptin is a 16 K-Da polypeptide hormone (167 amino acids). The OB gene codes for hormones are secreted mainly by adipose tissue, placenta, fetal tissue and membranes, and stomach. Leptin reaches the brain, crossing the blood-brain barrier or the chroroid-cerebrospinal fluid barrier and informs the brain about the size of the fat stores [1]. Leptin has a wide variety of central and peripheral actions such as reproduction, food intake, energy expenditure, lipid metabolism, immune system, blood pressure and angiogenesis [2, 3]. Recent evidence has shown leptin as a risk factor for vascular disease. It may be an important link between cardiovascular disease and obesity. Leptin has a procoagulnat, atherosclerotic role and platelet aggregation effect [4].
Homocysteine (Hcy) is a sulfur-containing amino acid produced from food methionine metabolism in the body. It can be converted to methionine or cysteine by remethylation or trans-sulforation cycles with some enzymes (MS, MTHFR, SAM) and cofactors (B6, B12). Folate as a methyl group donor is essential in the remethylation cycle, too. Hcy is transported from blood brain barrier into the CSF and brain, although human neural cells are capable of producing Hcy [5]. Insufficient folic acid, B6, B12 and impairment in enzymes functions cause hyperhomocysteinemia. Hcy>12 µM/dL has been shown as a risk factor for vascular disease, brain athrophy and neurodegenrative disease [6]. Hyper-homocysteinemia has been linked to atherosclerosis and thrombosis [7].
Ethanol has weakly charged molecules that move easily through the cell membrane, rapidly equilibrating between blood and tissue. Alcohol, at low doses can have some beneficial effects such as decreased rates of myocardial infarction, stroke, gallstone, and possibly vascular or Alzheimer’s dementia, but the consumption of more than 2 standard drinks per day increases the risk for health problems in many organ systems and hormonal changes [8]. Ethanol intake by changes in adipose tissue and BMI can affect leptin concentration [9]. Some studies have indicated an inverse relation between alcohol use and leptin level [10]. In other investigations ethanol is shown as a powerful inducer of hyperleptinemia in both animals and humans. Alcohol intake has the potential to alter body weight as it is energy-dense and may also alter eating behavior at higher levels of consumption. Each of these lifestyle factors, therefore, has the ability to alter adipose tissue mass, possibly via leptin [9]. On the other hand, short-term and chronic ethanol intake influences Hcy concentration by changes in the methylation pathway, folate and cofactors concentration [11]. Ethanol-induced increase in serum Hcy levels has been observed in active alcoholics. Exogenous ethanol caused elevated endogenous brain Hcy level, reduced S-adenosyl methionine (SAM) levels, and increased S- adenosyl Hcy (SAH) levels, which correlated to increased brain caspase-3 activities [12].
The reported sequences of leptin from human, cow, pig, sheep, mouse, rat, dog, and chicken showed a high degree of sequence conservation. This similarity suggests a common function or mechanism of hormone across species [13]. On the other hand, in previous studies exogenous ethanol caused a 1.6 fold increase in chick brain Hcy level at 11 days of development [12].
The present study was to investigate the effect of acute (70%) and chronic (10%) exposure to evaporated ethanol on brain leptin and Hcy concentration on the 15th day of embryonic development of chick, brain and serum leptin, and Hcy concentration immediately after hatch of chick. Fifteenth day that we used in present study was according to our previous study [14].
Materials and Methods
In this experimental study all procedures were approved by the animal care committee of the Iran academy of veterinary medicine. This study was fundamental experimental. Sixty fertile, pathogen-free, cub eggs were purchased from Fars Poultry center. More than 20 eggs (natural fertility is rarely 100%) of each group (acute, chronic exposure, control) were incubated under standard conditions (75% humidity at 37ºC). Humidity and temperature were checked by thermometer and a hygrometer. Incubator turning (3 times a day) was considered essential in the early stages. For the last 3 days of incubation when the chick was preparing to hatch, turning was stopped.
Test groups: eggs were divided into three groups 1- control group (eggs incubated in normal condition, N=20), 2- chronic group (eggs incubated while the water in incubator was replaced by 10 % ethanol, N=20), and 3- acute group (eggs incubated while the water in incubator was replaced by 70 % ethanol at the 6th, 13th and 20th days). In all groups half of the eggs were examined on the 15th day of developmental stage and the other half were examined immediately after hatching of chick.
For the extraction of brain, single whole brain was mixed with a phosphate buffer solution after homogenization, the mixture was centrifuged, and the brain extract was prepared for measurement of brain HCY and leptin. HCY were assayed by liquid stable 2-part HCY reagent cobas mira plus and leptin was assayed by chicken leptin ELISA kit from Cosabio Inc. The data were analyzed by SPSS-18 program and using one way ANOVA and Tukey as post hoc test. Significant level was considered to be p<0.05.
Results
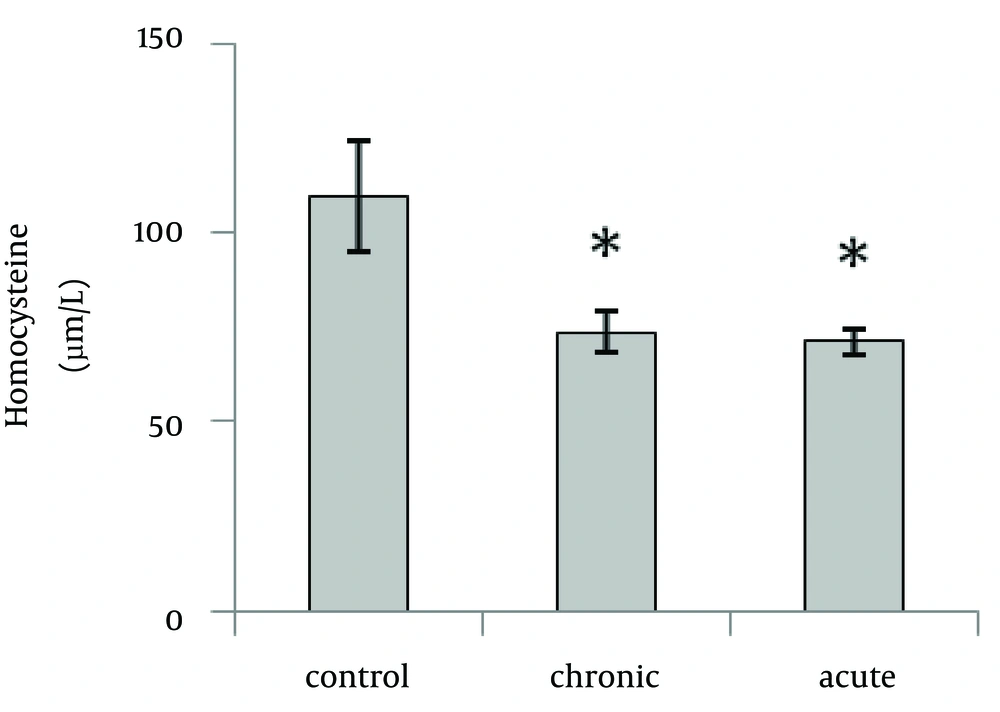
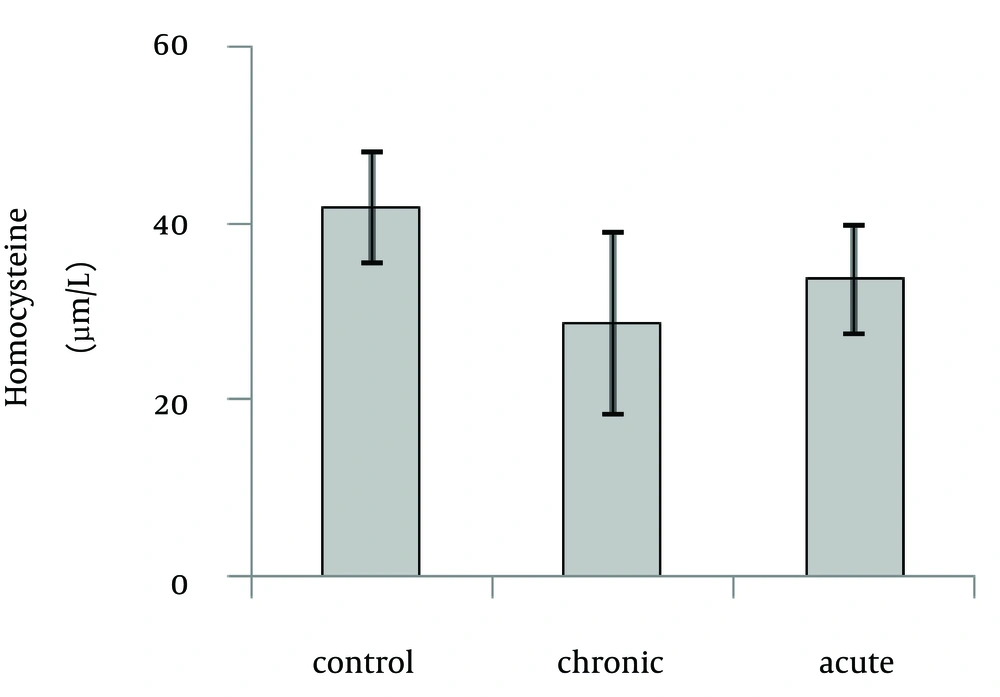
Our data showed that brain Hcy concentration significantly (p=0.01) decreased on the 15th day of embryonic stage of chicken in acute and chronic groups relative to control group (Fig. 1). Figure 2 shows that acute and chronic exposure to ethanol had no significant effect on Hcy concentration on the first day of hatch.
Brain Hcy concentration on the 15th day of embryonic stage and the first day of hatch had not significant difference between acute and chronic groups.
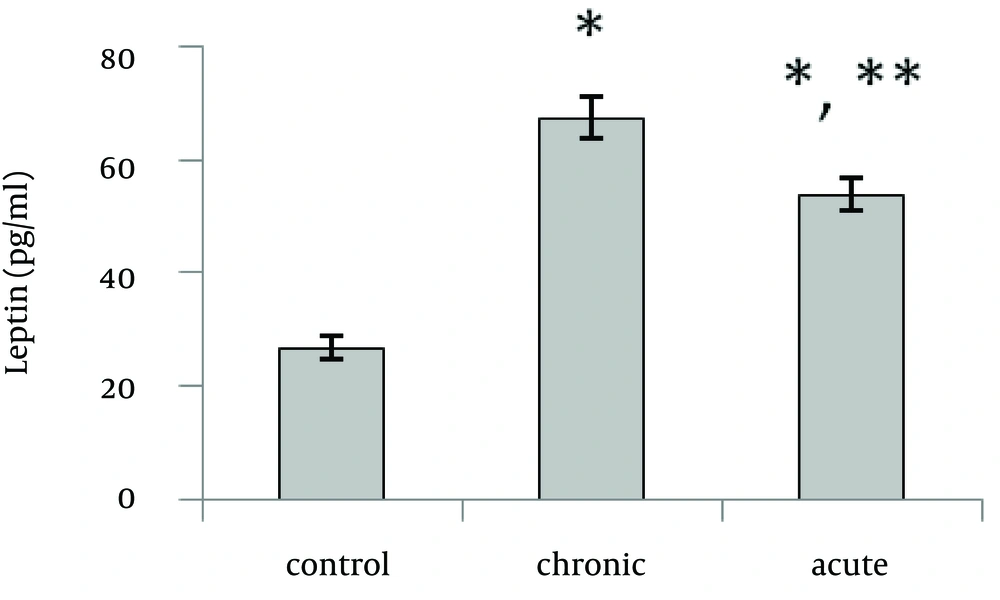
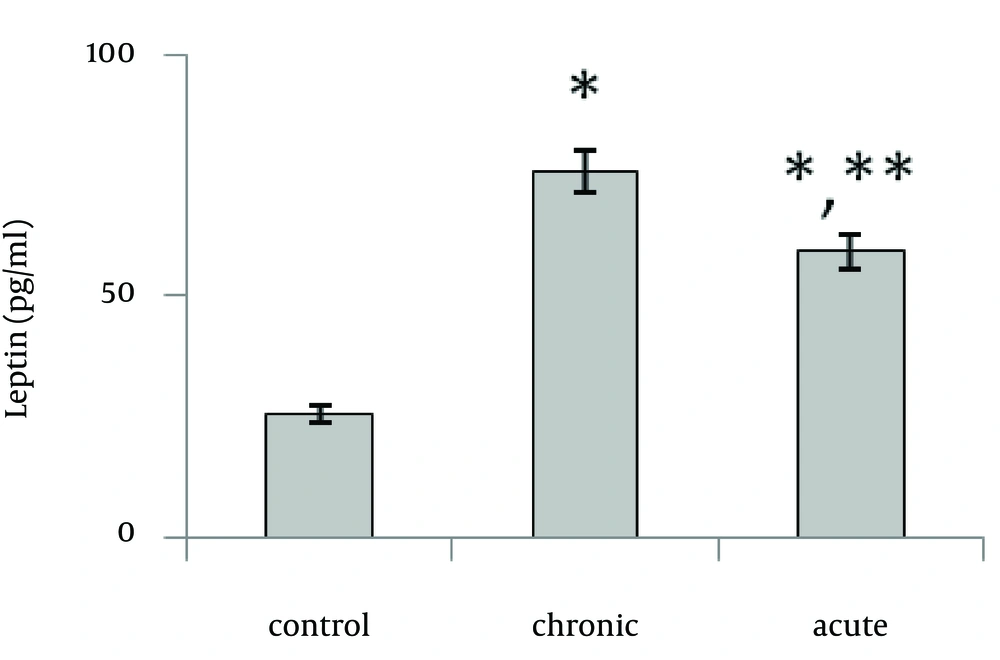
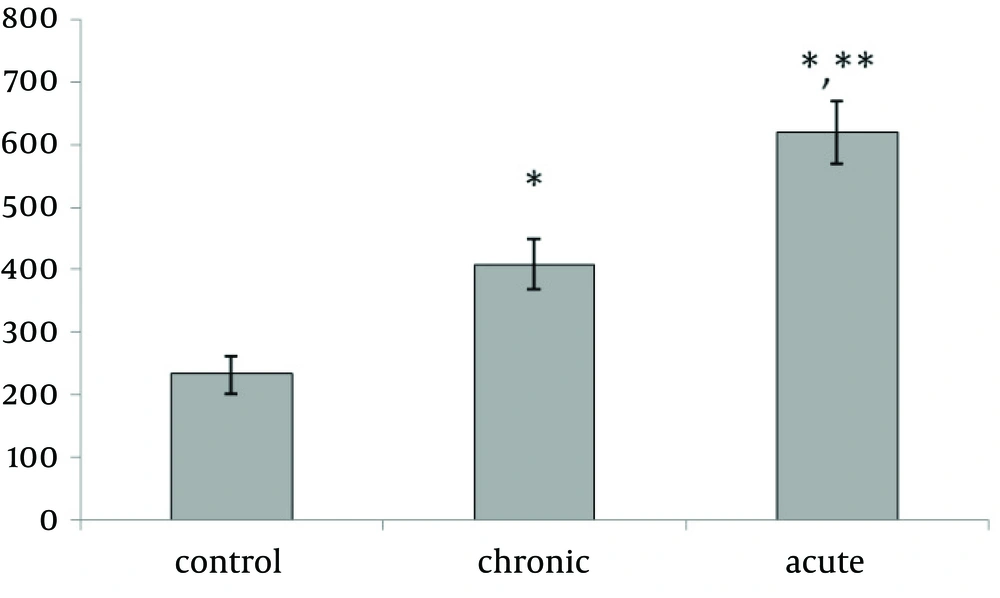
Present data showed that following acute and chronic exposure to ethanol, brain leptin significantly (p<0.001) increased on the 15th day of embryonic stage of chick and on the first day of chick hatching (Fig. 3, 4). Also, serum leptin significantly (p<0.01) increased following acute and chronic exposure to ethanol on the first day of chick hatching (Fig. 5).
Brain leptin concentration on the 15th day of embryonic stage and the first day of hatch, significantly was lower in acute than chronic exposure to ethanol. But serum leptin concentration on the first day of hatch was higher in acute than chronic exposure to ethanol.