Introduction
Alzheimer disease (AD), the most common cause of dementia, is usually divided into familial and sporadic forms, according to family history [1]. Three causative genes have been identified, alteration of which cause an autosomal dominant form of AD of young onset (familial AD) and usually is early-onset. AD is neuropathologically characterized by two types of brain lesions: neurotic plaques and neurofibrillary tangles [2]. The amyloid beta peptide, the main component of the neuritic plaques, is generated by beta and Gama secretas cleavage of the amyloid precursor protein (APP) [3]. Mutation in three genes, APP, presenilin 1 (PSEN1) and Presenilin 2 (PSEN2) are involved in the etiology of early-onset Alzheimer’s disease (EOAD).
Most cases of AD are sporadic and late-onset; a positive family history modestly increases AD risk. Some patients with EOAD have a family history consistent with autosomal dominant inheritance EOAD [4]. Since several missense mutations chiefly in exons 5 and 7 of PSEN1 gene and exons 5 and 6 of PSEN2 gene have been found associated with the early-onset form of familial AD (FAD) in other countries, so we restricted our investigation to these four exons [5].
Materials and Methods
In this experimental study 24 unrelated patients from Iran at 40 to 65 years of age, who were diagnosed based on stringent criteria in memory clinic of Rouzbeh hospital and Imam Hossein hospital.
Forty eight healthy subjects as control group were included in this study. Cross sectional method were used in this study. After DNA extraction with salting out method (Diatom DNA extraction kit, Gene Fanavaran, Iran) exons 5, 7 PSEN1 and exons 5, 6 PSEN2 genes were amplified by PCR-sequencing method. Primer sequences for PSEN1 and PSEN2 genes were ordered according to ones described by Marc Cruts [5]. PCR products were sequenced by ABI 3100 (KOWSAR company) and Finch TV software was used to analysis the DNA sequences and compared with gene bank data base (Gene Bank No. D87675). All patients informed and filed consent form. This research was comparative between the rate of mutation in Iran and other countries.
Results
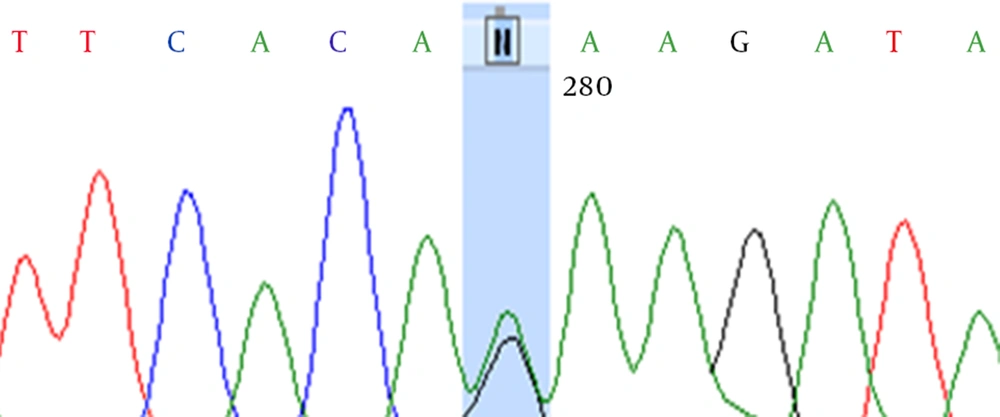
Fourteen women and 10 men with EOAD were investigated in this study. Two different mutations were detected in group, GAA62AAA (Arg 62 His) in exon 5 of two patients and CAG120CAC (Glu 120 Lys) in exon 5 of PSEN1 and PSEN2 genes respectively (Fig. 1).
Discussion
EOAD represents less than 5% of all cases with AD. Mutations in PSEN1, PSEN2 and APP genes are the main reason of EOAD with autosomal dominant inheritance. The number of EOAD patients with pure Alzheimer are low, so finding 24 patients in Iran was big problem, on other hand diagnosis of the disease at the age of 40-65 years was difficult (different reasons can lead to EOAD, for example: head damage, hysterectomy, smoking, …) Approximately 50% of EOAD is related to PSEN1 gene. Eighteen mutations were reported in PSEN2, whereas more than 180 mutations in PSEN1. In one study which was done by Piscopo et al., in France on 436,710 subjects, they showed that 100,000 of them were diagnosed EOAD from which 5.3% had autosomal dominant and their illness were diagnosed before the age 61 years [6].
In their study, 56% mutation in PSEN1 gene and 19% in APP gene was seen. Totally, rate of the mutation in PSEN1 is around 44% in PSEN1 and 3% in PSEN2 (Table 1). So in our study we expected to see mutation in 11 and 1 EOAD patients respectively in PSEN1 and PSEN2 genes, if all of the exons were analyzed (Table 1). Due to the expansion of the geographic and ethnic diversity in Iran, there are officially at least 20 ethnic groups; the results were not expected, on the other hand, because of the rarity of the disease (about 0.05%), since 50% of EOAD are associated with mutations in PSEN1 and PSEN2, consequently, the proportion of hot spots would be negligible. However, our early study showed that all genes in Iranian patients should be evaluated to identify specific mutations for any Iranian ethnic groups. Finding of a mutation in the family can help their families to have program for preventing. Preventive medicine will cause lack cell death in patients and it will delay the symptoms. The efficiency age of the family is usually at the 25-65. Preventing from appearance of the signs, not only guarantees the individual's health, but also helps the society to maintain its psychological, physical and economical health. Furthermore, it will decrease the huge costs of treatment and taking care of the patients that families and society pay.
| Gene | Mutations | Families |
|---|---|---|
| 32 (7.60%) | 89 (8.33%) | |
| 185 (43.94%) | 405 (37.92%) | |
| 13 (3.09%) | 22 (2.06%) |
Mutation per genes
The share of exons 5 and 7 of PSEN1 gene are around the 43% and exons 5 and 6 of PSEN2 gene are 46%. Thus, in our study we expected to have mutation in 5 patients, but only 3 of them had mutation in these 4 exons (Table 2, 3). According to the literature these exons were detected as hot spot for EOAD patients, but we could not find high level of these mutations in Iranian patients. So investigations of other exons of PSEN1 and PSEN2 genes are suggested.
| Exon | Mutations | Families |
|---|---|---|
| 38 (20.54%) | 93 (22.96%) | |
| 42 (22.70%) | 75 (18.52%) |
Mutation in PSEN1 gene
| Exon | Mutations | Families |
|---|---|---|
| 4 (30.77%) | 12 (54.55%) | |
| 2 (15.38%) | 3 (13.64%) |
Mutation in PSEN2 gene
For the first time Glu 120 Lys mutation was reported in PSEN1 gene by Hutton et al., in EOAD patients, and Arg 62 His mutation was detected by Cruts et al. in three patients. In their study 101 unrelated familial and 10 autosomal dominant EOAD (ADEOAD) patients were included. These two mutations are exactly pathogenetic and associated with ADEOAD [5, 7].