Introduction
Nitric oxide (NO) is one of the macromolecules which is synthesized in many cells types, including vascular endothelial cells, neurons, neutrophils, macrophages, platelets, fibroblasts and epithelial cells [1, 2]. It is one of the important signaling molecules which act as a regulator in several physiological processes in many tissues. This molecule is very small and able to pass cell membrane too fast. Firstly it was named EDRF (Endothelium-Derived Relaxing Factor). NO is a small gaseous molecule that has an important role in many biological processes like immune defense, neurotransmission, and the regulation of cell death (apoptosis), besides promoting blood vessel relaxation and regulation of vascular tone [3, 4].
NO is synthesized from L-Argentine by enzymes called nitric oxide syntheses (NOS). There are 2 is forms of NOS: cNOS (constructive nitric oxide syntheses) and iNOS (inducible nitric oxide syntheses). cNOS has been divided into 2 subdivisions: nNOS (neuronal NOS) and eNOS (endothelial NOS). It may present in epithelial cells too [5]. None and eNOS are calcium-dependent and responsible for NO production in nervous tissues and vascular endothelium in physiological conditions. While iNOS is a calcium-independent enzyme which produces NO in pathological conditions and is induced by bacterial endotoxines and cytokines secreted by macrophages, neutrophils, endothelium, smooth muscle cells and fibroblasts [6]. NO is an inhibitory noradrenergic and noncholinergic neurotransmitter that acts as an intra and intercellular signaling molecule in gastrointestinal and vascular smooth muscle. It is a very important mediator in most of physiological and inflammatory processes and causes relaxation in gastrointestinal smooth muscle. Gastrointestinal smooth muscle relaxation as a result of neuronal stimulation has been reported in rat, pig and dog. Moreover the role of NO in protection of mucosa and hemodynamic responses to hepatic diseases has been studied [2, 7].
NO may be a probable mediator in fluid and electrolyte transmission because of its effect on intestinal epithelium, blood circulation, enteric nervous system and inflammatory reactions [8]. The intestine is an important part of gut because of food absorption which takes place in entrecotes. Therefore injuries and constructive changes will affect on absorption process and damaged intestinal epithelium loses its function and leads to malabsorption syndromes [9]. Considering the effects of NO on blood circulation and nervous system of intestinal epithelium and absorption of food and fluids, this study was done to evaluate the effects of L-Argentine as a NO precursor and L-NAME (L-NG-Nitro Argentine Methyl Ester) as a NO inhibitor on epithelial cells of jejunum.
Materials and Methods
This study is an interventional experimental research. A total of 40 adult female Sprague Daley rats aged 8 weeks, weighing 200-250 g were purchased from animal house of Tehran University of Medical Sciences. All animal experiments were carried out according to the guidelines of the Iranian Council for Use and Care of Animals and were approved by the Animal Research Ethical Committee of Tehran University of Medical Sciences. The rats were permitted free access to food and water at all times and were maintained under 12 h light (07.00-19.00)/12 h dark (19.00-07.00) cycle. Rats were randomly allocated to 5 groups of 8 animals. The first group served as the control with no injection. The second to fifth group received 2 mL/kg normal saline, 200 mg/kg L-Argentine, 20 mg/kg L-NAME, and a mixture of 2 substances for L-Argentine and L-NAME group intraperitonealy for 3 days respectively [10].
Defined dose of L-Argentine and L-NAME dissolved in 2 mL normal saline to prepare the solution [10, 11]. Two weeks later the rats were sacrificed and Jejunum of animals was removed. Tissue samples were fixed in 10% formalin, dehydrated through graded alcohols, and cleared in xylene. Tissues were infiltrated and embedded in paraffin. Finally prepared 5-6 micron thickness sections. The specimens were stained with hematoxylin and Eosin (H&E) and observed under light microscopy (Olympus, Japan) [12]. For quantitative study 5 fields were randomly chosen from each of the 5 groups. Numbers and height of entrecotes was measured using Image tools III Microsoft software. Analysis was made by SPSS-16 software and One-way ANOVA followed by Tukey post hoc test to evaluate the statistical significance between different groups. Data are expressed as the means. A value of p<0.05 was considered statistically significant.
The experimental protocols were consistent with the guide for the Care and Use of Laboratory Animals published by the NIH (National Institute of Health).
Results
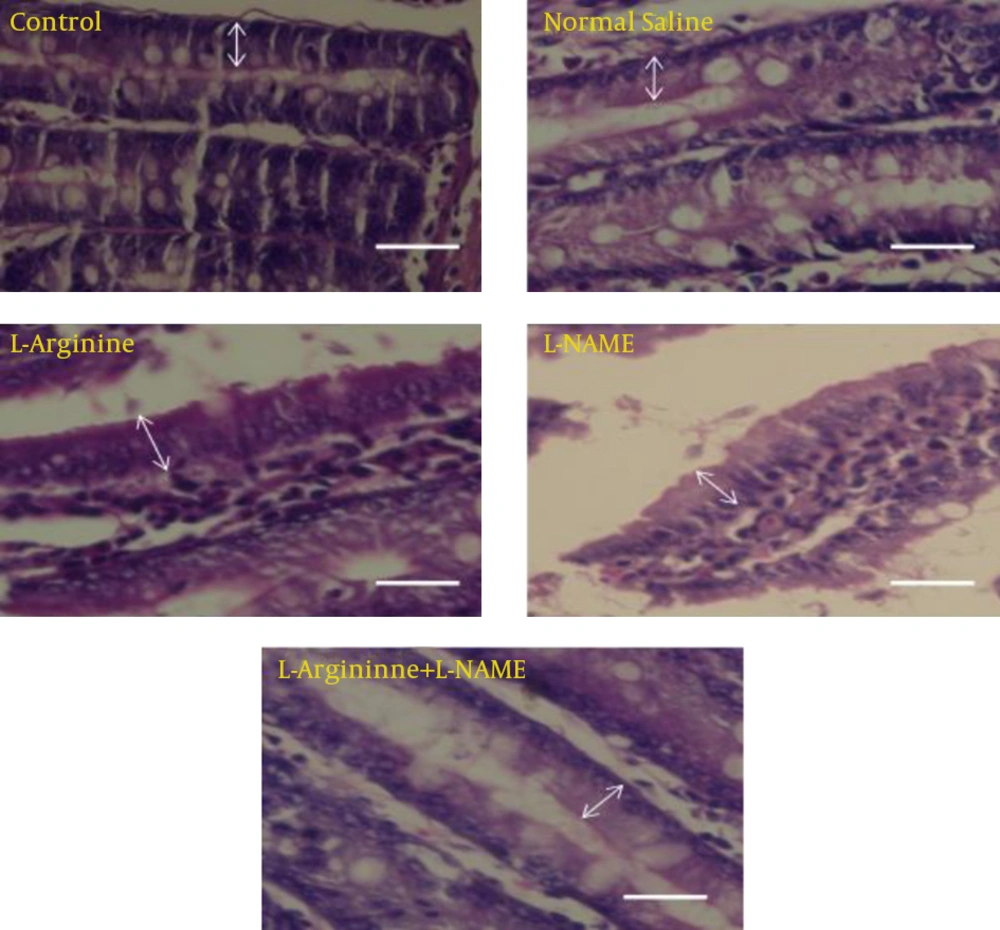
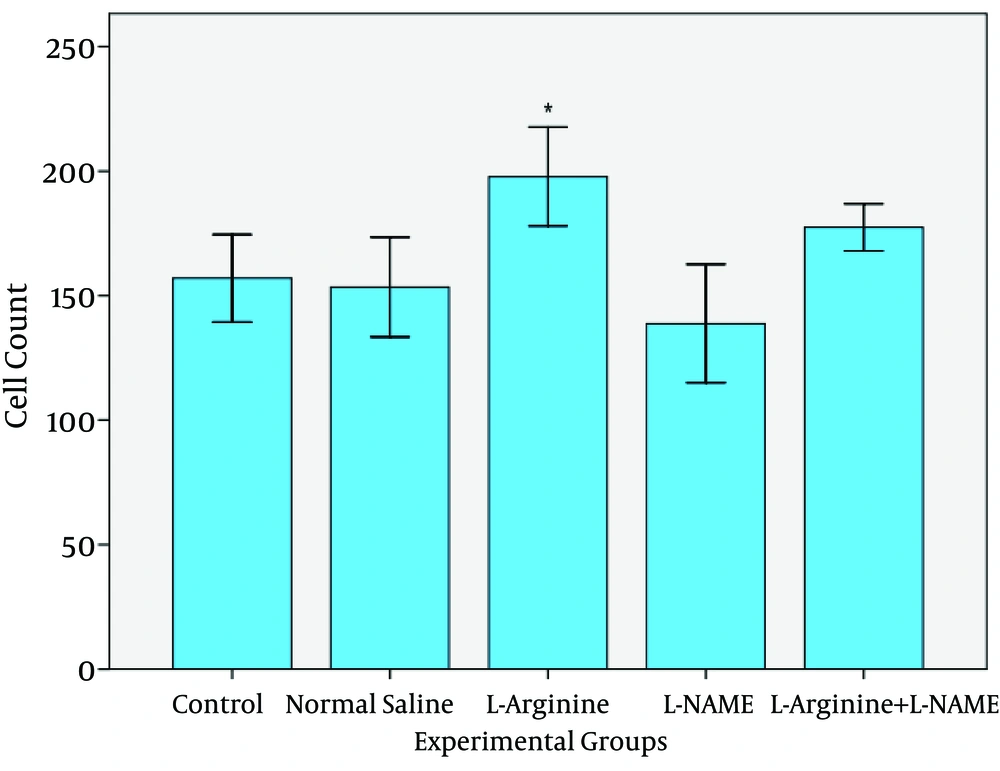
Photomicrographs were taken from different groups (Fig. 1). Table 1 and figures 2 and 3 shows the result of number and height of epithelial cells of jejunum. The results show that the number of entrecotes in jejunum was significantly higher in L-Argentine group than four other groups (p=0.024). Cell number was decreased in L-NAME group in comparison with control, normal saline and L-Arginine+L-NAME groups. But it was not statistically significant. In spite of increased cell number in L-Arginine+L-NAME group compared with control and normal saline group, no significant difference was detected (Fig. 1, 2).
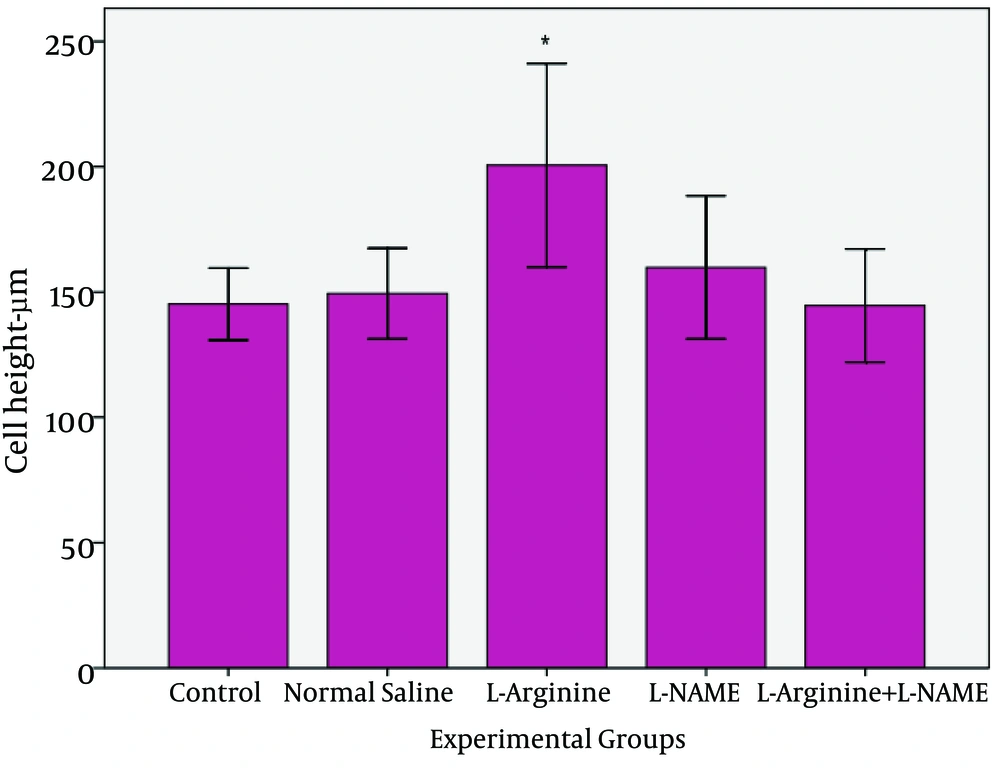
The results of morphometric evaluation in this study showed that height of entrecotes of jejunum in L-Argentine group was significantly increased compared with control, normal saline and L-Arginine+L-NAME groups (p=0.045). Whereas there was no significant difference with L-NAME group (Fig. 1, 3).
Comparative photomicrographs of different groups which show epithelial cells of jejunum and their morphological differences. Cell’s height in L-Argentine group compared with other groups was higher and as the photomicrograph shows, epithelial cells are in a compact arrangement. In L-NAME group cell number decreased and cell height increased in comparison to control and normal saline groups. But no significant difference was detected. While in L-Arginine+L-NAME group cell number increased and cell height decreased compared to control and normal saline groups. Arrows show height of cells (Hematoxylin & Eosin staining, 400X). Scale bar: 20 µm
| Control | Normal saline | L-Arginine | L-NAME | L-Arginine+L-NAME | |
|---|---|---|---|---|---|
| 157±19.6 | 153±22.3 | 197.8±22.1 | 138.8±26.6 | 177.4±10.5 | |
| 145.2±16 | 149.4±20.2 | 200.6±45.4 | 159.8±31.8 | 144.6±25.2 |
Discussion
In this study, effect of L-Argentine as a NO precursor and L-NAME as a NO inhibitor on epithelial cells of jejunum was evaluated. The results demonstrated that L-Argentine and L-NAME administration caused increase and decrease of entrecotes number respectively. The height of epithelial cells did not have any significant difference between L-Argentine and L-NAME groups while a significant increase was observed in L-Argentine group in comparison with three other groups.
Effects of NO on enteric blood circulation and its protective role on intestinal mucosa were shown in 1992 [7]. Then several studies were done and the researchers introduced it as a mediator of intestinal fluid and electrolyte transport [8, 13]. In recent studies, NO has been introduced as a vascular regulator which increases intestinal blood circulation and stimulates cell proliferation, protein synthesis and cell survival [6, 14, 15]. It has been demonstrated in many researches that NO is found in gastrointestinal vessels and endothelial myentric nerves. NO removes free oxygen radicals from digestive system. Moreover, it inhibits inflammation and improves mucosal blood flow and permeability which prevents injuries to intestinal mucosa [16, 17]. NO protects epithelium against damages and also play an important role in epithelial reconstruction [18]. In a research which was focused on the effect of NO on superior mesenteric artery (SMA) blood flow, the researchers reported that decrease in NO level causes vasoconstriction and defect in intestinal blood circulation which lead to intestinal mucosal atrophy [19]. Another study represents that hypoxia results in intestinal ischemia because of decrease in SMA blood flow. Hypoxia and ischemia have physiological and pathological effects on integrity of epithelial barrier of intestine. NO improves mucosal permeability and acts as a noradrenergic and noncholinergic mediator to improve vascular tonicity. NO also protect intestine against toxins, and prevent apoptosis and necrosis, leading to survive epithelial cells and decrease mortality [20].
Studies show that high concentration of NO was effective in progress of colon cancer in mouse and in pathogenesis of celiac disease. On the other hand, it has been reported that L-NAME (NOS inhibitor) induces decrease in cell proliferation of colon. Therefore, researchers suggest that it may be used as a prophylactic factor for colon cancer [21-23]. Our results are consistent with the findings of mentioned studies. In the current study, entrecote number of jejunum in L-NAME group was significantly decreased in comparison with L-Argentine group. But there were no significant difference in cell height between L-NAME group and other groups. Moreover, cell number in L-Argentine group was significantly increased. This suggests that NO can be considered as a mitotic factor which plays a role in intestinal cells proliferation [21]. On the other hand, it has been reported that because of its mutagenic effect, NO can act as a protective factor against gastrointestinal ulcers induced by bacteria, stress and drugs like NSAIDs [6, 15, 24]. There are inconsistent reports about the effects of L-NAME on intestinal epithelial cells. However, the results of the present study are in contrast with Akgun-dar et al. observations. According their report, L-NAME prescription induced significant increase in the height and proliferation of intestinal epithelial cells [24].
The present study demonstrated that after NO precursor administration, the number and height of entrecotes increased in comparison with control, normal saline, and L-Arginine+L-NAME groups. It may be attributed to NO synthesis stimulation and the effect of NO on modulation of intestinal blood flow. Whereas L-NAME group had no statistically difference with control and normal saline group. L-NAME ineffectiveness may be related to its dose which could not inhibit any synthesis. In L-Arginine+L-NAME group the results were the same as normal saline and control groups. But there is a question: is the increase in cell number consequence of mitosis enhancement or is due to decrease in apoptosis? On the other hand it has been shown that celiac and malabsorption diseases as well as radiation induce entrecote injury will result in decrease of entrecote height [9, 25, 26].
In the present study, L-Argentine increased entrecote height. It prevented cell damage which is due to improved jejuna blood flow. While there was not any significant difference in L-Arginine+L-NAME group compared to control and normal saline groups. In conclusion, the results of this experiment demonstrated that NO and its precursor increase number and height of jejuna entrecotes. Further studies are required for investigating the exact mechanism of NO and L-Argentine on the number and height of intestinal epithelial cells. Therefore, besides stereological and morph metrical studies, assessments of apoptosis, evaluation and measurement of serum level of eons and different doses of NO precursor and inhibitor is suggested.