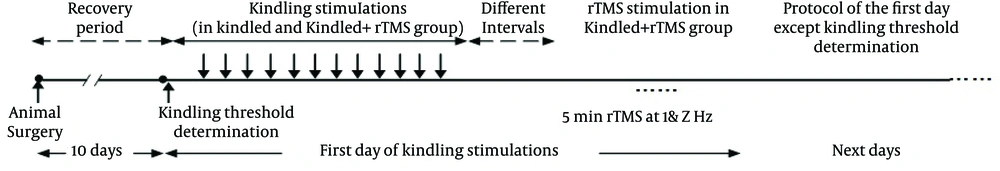
Introduction
Epilepsy is a neurological disease from which about 1.5% of global human populations are suffering. This disease is categorized based on various electrographic recurrent attacks. The representation of these attacks lies in the brain and progressively spread from seizure origin to different areas, and even whole brain, electrophysiologically. The varieties of this disease share a single feature: epilepsy increases the cortical excitability of the brain [1]. Temporal lobe is the most prevalent epilepsy [2]. Today, anticonvulsive medications are largely used to tackle epilepsy; however, about 20% of epileptics are drug-resistant [3]. In such cases, surgery is used. Nevertheless, surgery is an invasive method that in several cases is accompanied with irreversible side effects, leading to neuronal damages [4].
The previous studies have shown that low frequency rTMS can have therapeutic effects on epilepsy-affected laboratory models. For example, Akamastsu et al. showed that rTMS of 1000 pulses at 0.5 Hz led to a prolonged latency for seizure development and decreased frequent seizures after intraperitoneal injection of pentylenetetrazol in Wistar rats [5]. Roteaberg et al. investigated antiepileptic effect of rTMS on kainic acid epilepsy model at 0.75, 0.5, and 0.25 frequencies. They found out that seizure duration decreased at 0.5 and 0.75 Hz frequencies, but 0.25 Hz frequency had no impact on it [6].
These observations demonstrate that anticonvulsive effect of rTMS depends on its frequency. Kindling is a standard model for investigation into the impacts of different therapeutic factors on epilepsy and its resulted seizures [1]. Applying physical parameters such as using 50 Hz magnetic field has shown a weak inhibitory influence on seizure process of kindling [7]. Even though, no report of the rTMS effects, with different frequencies and intensities, on neural excitability parameters, in seizure state, has been published.
Inhibitory and stimulatory effects of rTMS depend on field intensity, duration of the applied pulse train, time interval between each pulse train and applied frequency in particular [7-9]. Due to the huge advantage of this technique (rTMS is a painless and cheap procedure without side effects), obtaining its optimal parameters, especially the applying frequency, can bring beneficial results in considering this method as a supplement or an alternative for conventional epilepsy treatment technique that are with several side effects. In this study, the therapeutic impacts of 1 and 2 Hz rTMS on convulsing parameters in epileptic model of electrical kindling stimulation of the perforant path were investigated.
Results
There was no significant difference between mean subsequent discharge wave duration after first stimulation and stimulation threshold intensity in animals in different groups. That is, the intensity of neural system excitability was equal in different groups. Applying kindling stimulation to kindle group caused animals to reach fifth stage of convulsion after 5.4±0.5 days, in average. The intensity of anticonvulsive effects of different rTMS frequencies in various studies was compared with the data from this group of animals over 7 days.
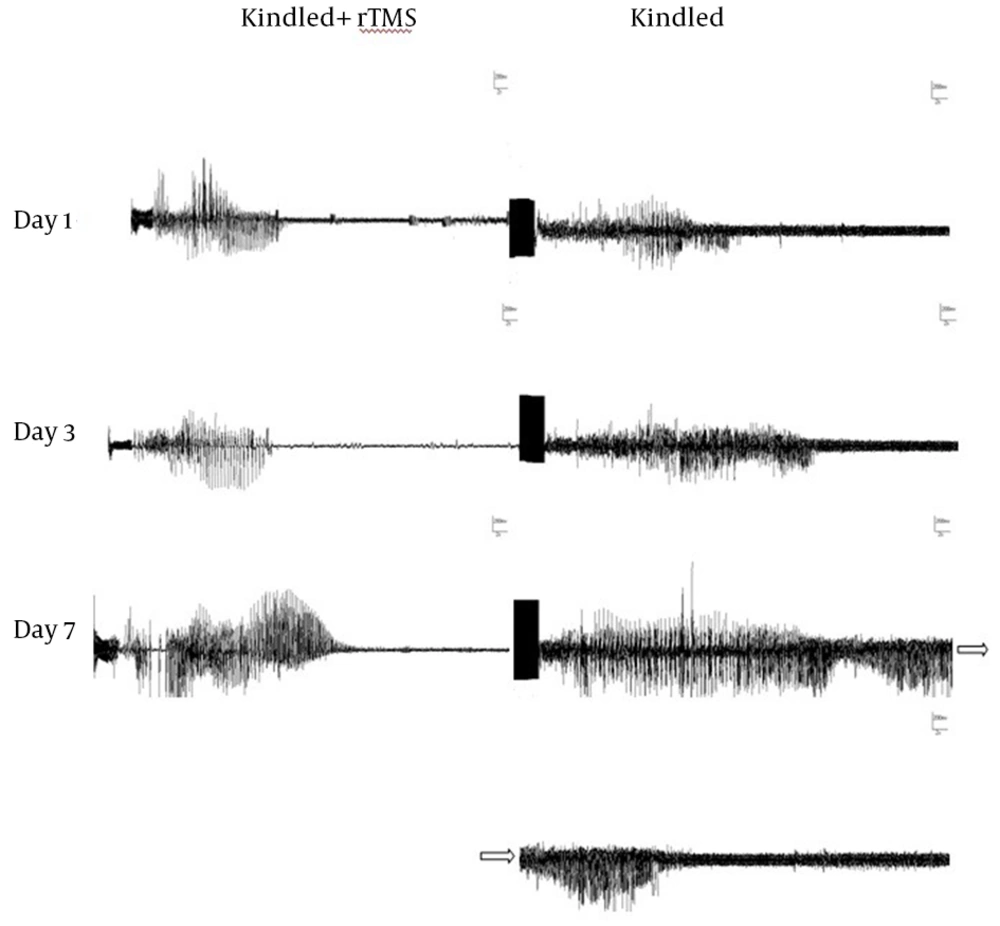
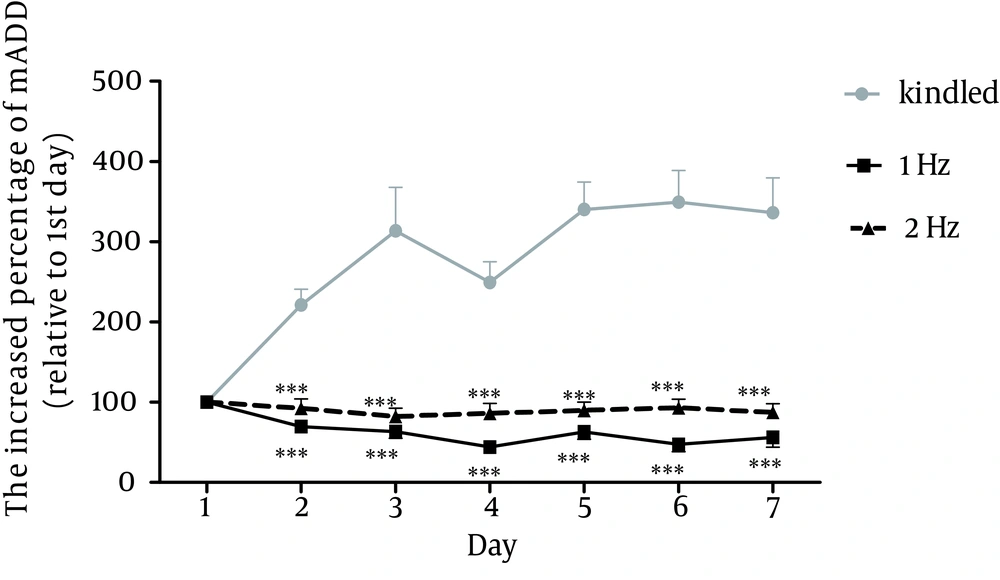
In order to investigate the impact of rTMS frequency on the intensity of anticonvulsive effects, 1 Hz and 2 Hz rTMS were applied. A sample of subsequent discharge waves of kindle and kindle+rtms groups are presented in figure 2. The comparison of mADD was performed using two-way ANOVA test in 7 days between kindle and kindle+rTMS groups, receiving rTMS at different frequencies. The results indicated significant difference in that regard between the mentioned groups (p<0.001). Bonferroni subsequent test showed a significant difference between kindle+rTMS group at 1 Hz and 2 Hz frequencies and kindle group. However, there was no significant difference between 1 and 2 Hz group (Fig. 3).
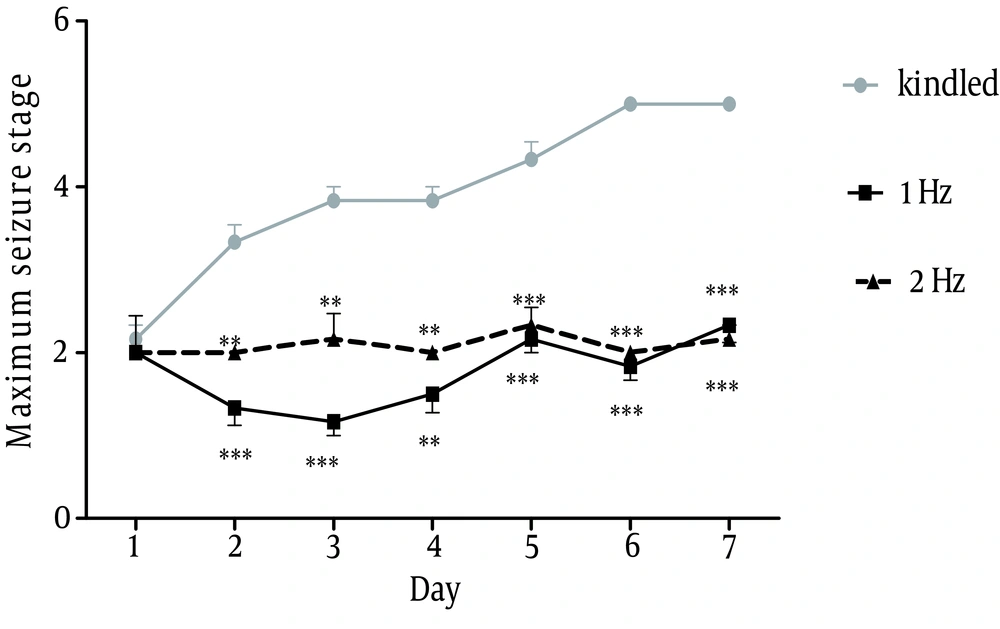
Comparison between behavioral phases of convulsion revealed a significant difference between groups receiving 1 Hz and 2 Hz rTMS and kindle group, using Kruskal-Wallis test. Mann-Whitney U test showed a significant difference between kindle+rTMS and kindle groups from the 2 day. However, there was no significant difference between the two rTMS groups, p<0.001 (Fig. 4).
Sample of discharge waves after Kindling group and Kindling + rTMS group. (Kindling stimulation was applied for 2 seconds at the start of registration and scale of all these figures were for 2 seconds for the horizontal axis and 200 mV for the vertical axis that are not clear due to lack of space)
Discussion
The findings of this study showed that applying rTMS at 1 and 2 Hz frequencies has remarkable impact on inhabitation of convulsive stages. In that, this anticonvulsive effect was observable from the first day after applying rTMS. In addition, those animals that had received rTMS did not show fourth and fifth convulsive stages, and the highest discharge wave observed in them was with a considerable reduction compared with kindle group. This study was in consistent with previous ones. For example, Akamatsu et al. demonstrated that applying 1000 pulse of rTMS at 0.5 Hz frequency caused mitigation of epileptic seizures in successive epilepsy model, after injection of pentylenetetrazol to peritoneum of epileptic rats [5]. Rotenberg et al., revealed that rTMS at 0.5 and 0.75 Hz has anticonvulsive effects on rats with kainik acid-induced epilepsy [6]. Ke et al. and Huang et al. studied the frequency-depended effect of rTMS in pilocarpine epileptic model. They applied rTMS to the rats at different frequencies for two weeks. Then, pilocarpine was injected and it was observed that 1, 0.8, 0.5, and 0.3 Hz frequencies postponed the onset of seizure [12, 13]. It has been demonstrated that low frequency stimulations generate antiepileptic effect through decreasing NMDA receptor and subsequent reduction of neuron excitability [14, 15].
In a preliminary study by Anschel et al., it was demonstrated that 1 Hz rTMS has anticonvulsive effect [16]. In the mentioned experiment, the injection of cerebrospinal fluid of depressed patients, exposed to 1 Hz rTMS, to the ventricle of the rats decreased kindling rate through increasing seizure threshold. rTMS was stimulated for eight daily sessions of 26.6 min. The intensity of stimulation was equal to 90% of individual’s motor threshold. The same protocol was employed for 10 Hz rTMS. A total of twenty pulse trains, each for 8 seconds with 22 seconds interval and total radiation duration of 22 min were applied. The results showed that in contrast to 1 Hz frequency, 10 Hz frequency did not reduce seizure threshold. These findings conform to the results from the present study in that 1 Hz rTMS inhibits and suppresses neuronal activity. However, duration of stimulation and also the type of protocol (injection of cerebrospinal fluid of radiated sample to the studied sample) were different from the present study. Regarding the above, it could be argued that rTMS radiation causes secretion and increased concentration of endogenous anticonvulsive substances in cerebrospinal fluid. These cases possess anticonvulsive effects and increase in their concentration can last for several minutes after rTMS radiation [16]. It has been suggested that the time-varying magnetic field at some frequencies and intensities cause release of melatonin, an anti-epileptic compound, in the brain tissue [17, 18].
There are other theories regarding the mechanism of this technique. Modulating the voltage-dependent sodium ion channels [19, 20] and the release of neurotransmitters at interneuron in cortical and cerebrospinal regions [21, 22] are among the mechanisms of the drugs, with anti-convulsive effects on rTMS variables suggested for the patients with different types of epilepsy and normal samples. However, due to the fact that all generated physical and physiological effects of this technique on brain tissue and intranetworking impacts of different points of brain on each other are not quite determined, then the mechanism produced by this technique in the brain is not exactly obvious.
In order to establish this promising technique as an alternative or supplementary option for curing treatment-resistant epilepsy, on the one hand, the exact simulation of physical parameters of generated electric field in the brain tissue and performing experiments at molecular level can be helpful in clarifying theses mechanisms. On the other hand, further studies to investigate the effects of other frequencies, to obtain optimal frequencies with therapeutic impacts, and to study the effects of other parameters of this technique such as intensity of magnetic stimulation radiation, spatial shape of radiation coils, duration of magnetic stimulation, and spatial distance and direction of radiation coil can help determine the optimal radiation conditions for curing treatment-resistant epilepsies. Due to the advantages of this technique, obtaining optimal parameters for treating epileptics can be a step towards it’s clinically popularization.