Introduction
Leukemia inhibitory factor (LIF) is a glycoprotein, categorized as a subfamily of interleukin 6 cytokines with a wide biological function range, and numerous effects on target cells [1]. The human life gene is located on chromosome 22q12.2 containing 3 exons. This gene produces a 3935bp long transcript that codes for a protein with 202 amino acids [2]. The LIF protein in mammolals is highly basic and has heterogeneous weight range, because of different glycosylation pattern. LIF firstly was isolated from fibroblasts and is characterized by its ability to induce differentiation of mouse myeloid leukemic M1 cells into macrophages [3]. The IL-6 subfamily cytokines, with a wide range of physiological functions in diverse fields, such as inflammolation, immolune responses, and cell survival including LIF, IL-6, IL-11, ciliary neurotrophic factor (CNTF), cardiotrophin-1 (CT-1), oncostatin M (OSM), and cardiotrophin-1 [4]. LIF has been shown to retard progression of motor neuron disease in a murine model of this disorder [5]. The action of LIF on nerve cells has also been examined in animal models. Directapplication of LIF to sites of nerve transaction improved survival of both sensory and motor neurons and LIF regulates neuron survival [6]. Nerve transection has been shown to increase expression of LIF and IL-6, and is associated with retrograde axonal transport of LIF [7]. In LIF-/- knockout mice have been seen that regulates blastocyst implantation and also acts as pre-inflammolatory cytokine that regulates immolune response [8]. These cytokines have some overlapping biological activities acting through receptors that share a commolon signaling molecule; the glycoprotein 130 (gp130) subunit [9]. The receptor for LIF protein is a heteromeric complex consisting of gp130 (also named LIFRα) and the LIF receptor (LIFR, also referred as to LIFRβ) [10]. The human LIF has some other known functions like induction of acute phase proteins [11, 12] and is important in the embryo development [13-15]. In previous study, cloning and initial expression of recombinant human leukemia inhibitory factor by pET-28a (+) was successfully done in E. coli strain BL21 [16]. The LIF gene accompanied with his-tag sequence in carboxyl end serves as a signal for easy and one step purification. The rhLIF was expressed and proved by SDS PAGE and Western blotting. In present research we optimized inducer concentration, inducing temperature and inducing time to reach highest amount of soluble recombinant protein to prevent inclusion body formation. Although purified rhLIF and proved it's purification by SDS PAGE and Western blotting.
Materials and Methods
Bacteria culture and inducing by isopropyl β-D-1-thiogalactopyranoside (IPTG): In this experimental study, for bacteria culture every time we added 4 μL of bacteria permanent culture including pET28 (+) to 10 mL of Luria broth (LB) culture including kanamycin (final concentration of 30 μg/mL, pET 28(+) includes resistance gene for kanamycin) and incubated for overnight in shaker incubator in 37ºC for 12-14 h in 150 rpm. After this time 8 μL of this medium added to 500 mL fresh culture with 30 μL/mL kanamycin and incubate in 37ºC and 150 rpm until reach OD600: 0.8 (approximately 3 hours). During incubation OD600 checked by spectrophotometer. After reaching desired OD600 cultures induced by 2 concentration of inducer including 0.2 mmol and 0.4 mmol and then incubated in 25, 30 and 37ºC in 150 rpm for 3, 4, 5, 6 and 7 h. We used auto induction medium includes lactose for comparing culturing results. Lactose is weaker inducer in contrast to IPTG for T7 promoter; auto induction medium includes lactose as inducer which will omit some steps such as OD600 measuring and addition of IPTG therefore the possibility of contamination by aero cells will reduce. In auto induction medium 1st source of energy for bacteria is glucose and second is glycerol and the last one is lactose which makes bacteria to run T7 promoter. Auto induction medium includes 6 g Na2HPO4, 3 g KH2PO4, 20 g tryptone, 5 g yeast extract, 5 g NaCl which should be autoclaved and 60% glycerol, 10% glucose and 8% lactose should be filtered first by 0.22 μ and then be added.
Final concentration of kanamycin should be at last 100 μg/mL, because excess phosphate causes kanamycin resistance [17].
Enzymatic and mechanical lysis of bacteria: Induced cells were collected by 4000× g, centrifuge for 15 min. Before sonication of cells we added 5-10 mL of binding buffer (20 mmolol sodium phosphate, 500 mmolol NaCl, 20 mmol imidazole, pH=7.4) for each gram of cell paste. Dissolved cell paste in binding buffer and added enzymatic agents to start enzymatic digestion including 0.2 mg/mL lysozyme, 1 mmol MgCl2, 1 mmol PMSF (final concentrations). Stirred for 30 min at room temperature on rocker in 40 rpm [18].
After stirring samples transferred on ice and sonicated for 4-6 times in maximum pulse, each time for 30 seconds. We run sonication until samples lose their viscosity and get transparent. Finally samples transferred to micro tubes and centrifuged in 14000 rpm for 10 min and discard cell paste and transferred supernatant into chromatography column.
Chromatography: Chromatography done according to factory instruction, briefly at first the column was equilibrated with 10 mL binding buffer then samples were added and washed with 10 mL binding buffer, applied 3 mL elution buffer (20 mmol sodium phosphate, 500 mmol NaCl, 500 mmol imidazole, pH=7.4) and collected the eluate, and used for furtherstudy.
Protein Expression Analysis: Expression of the hLIF was evaluated by SDS-PAGE at first, after assuring of expression, proceed to purification. We used SDS PAGE in twice: 1- before purification 2- after purification. The prepared samples and protein molecular weight marker (Fermentas) was heated at 90ºC for 5 min. Afterward each sample and marker was loaded on the 12% SDS-PAGE gel and run on the constant voltage of 120 V for 1 h. In second stage we took more samples for running on SDS PAGE gel, because measured concentration of purified rhLIF by spectrophotometer at 280 nM indicated low level of recombinant protein (80 uL sample+16 uL sample buffer). The gel was stained with Coomasie brilliant blue R-250 (Sigma) for 2 h and destained with the solution containing 450 mL methanol, 100 mL acetic acid (Merck) and H2O for first stage but for second stage we used some more sensitive methods to staining the gel. Silver staining and colloidal Coomasi brilliant blue R-250 were used, these method even respond for low concentration of protein, sensitivity of these methods are 100-1000 ng/band. Following electrophoresis placed the gel in the colloidal coomassie staining solution (Mix 16 mL ortho-phosphoric acid in 768 mL of distilled water. Add 80 g of ammolonium sulfate to this solution; prepare a solution of 5% CBB G250 in distilled water. Add 16 mL of this mixture to the solution prepared in step 1, immolediately before use, slowly add 200 mL methanol to the solution to give a final concentration of 0.08% CBB G250/1.6% ortho-phosphoric acid/8% ammolonium sulfate/20% methanol). Incubate the gel for 6 h to overnight in the staining solution. Then destained the gel with several changes of distilled water until the background is transparent, and silver staining was either. Fractionated proteins by SDS-PAGE from chromatography result, after assuring of protein band existing by Ponceau S staining and distaining, transferred to PVDF membrane (Millipore) by electro elution at 60 V for 3 h. The PVDF membrane was incubated overnight in blocking buffer [5% w/v skim milk (Merck) in 0.05% v/v PBS-Tween-20 solution] at 4ºC, followed by washing 3 times with PBS-Tween-20 and shaking in 50 rpm for an hour in 1:200 dilution of mouse monoclonal LIF antibody (Santa Cruz biotechnology, Inc.), with repeating washing step 3 times with PBS-Tween 20. The membrane was then incubated with 1/2000 dilution of a goat anti-mouse IgG peroxidase conjugate (Sigma) and the signal was detected by 4-chloro-1-naphthol (Biogen, Iran) substrate solution [16].
Results
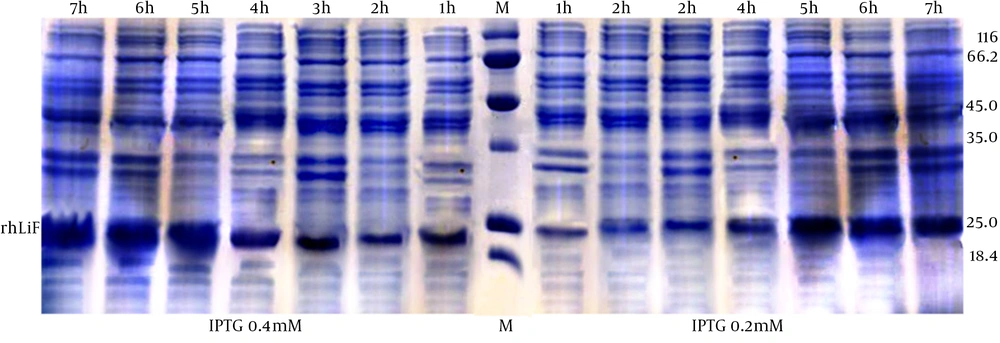
Bacteria culture and induction by IPTG: In contrast to previous study, conditions such as the temperature of 25ºC and the induction in 0.4 mmol concentration for 3 h resulted in more robust soluble recombinant protein and therefore more concentration of rhLIF. In current study we tried to optimize all factors, to gain high concentration of purified recombinant protein. rhLIF expression checked by SDS PAGE electrophoresis in 0.2 mmol and 0.4 mmol concentration of IPTG in different induction times (Fig. 1)
| Induction time | IPTG 0.4 mmol | 3 h | 4 h | 5 h | 6 h | 7 h |
|---|---|---|---|---|---|---|
| 25ºC | 0.528 | 0.42 | 0.361 | 0.282 | 0.255 | |
| 30ºC | 0.24 | 0.2 | 0.2 | 0.156 | 0.115 | |
| 37ºC | 0.223 | 0.218 | 0.182 | 0.114 | 0.101 | |
| IPTG 0.2 mmol | 3 h | 4 h | 5 h | 6 h | 7 h | |
| 25ºC | 0.192 | 0.171 | 0.186 | 0.18 | 0.145 | |
| 30ºC | 0.19 | 0.144 | 0.142 | 0.101 | 0.111 | |
| 37ºC | 0.175 | 0.161 | 0.148 | 0.15 | 0.092 |
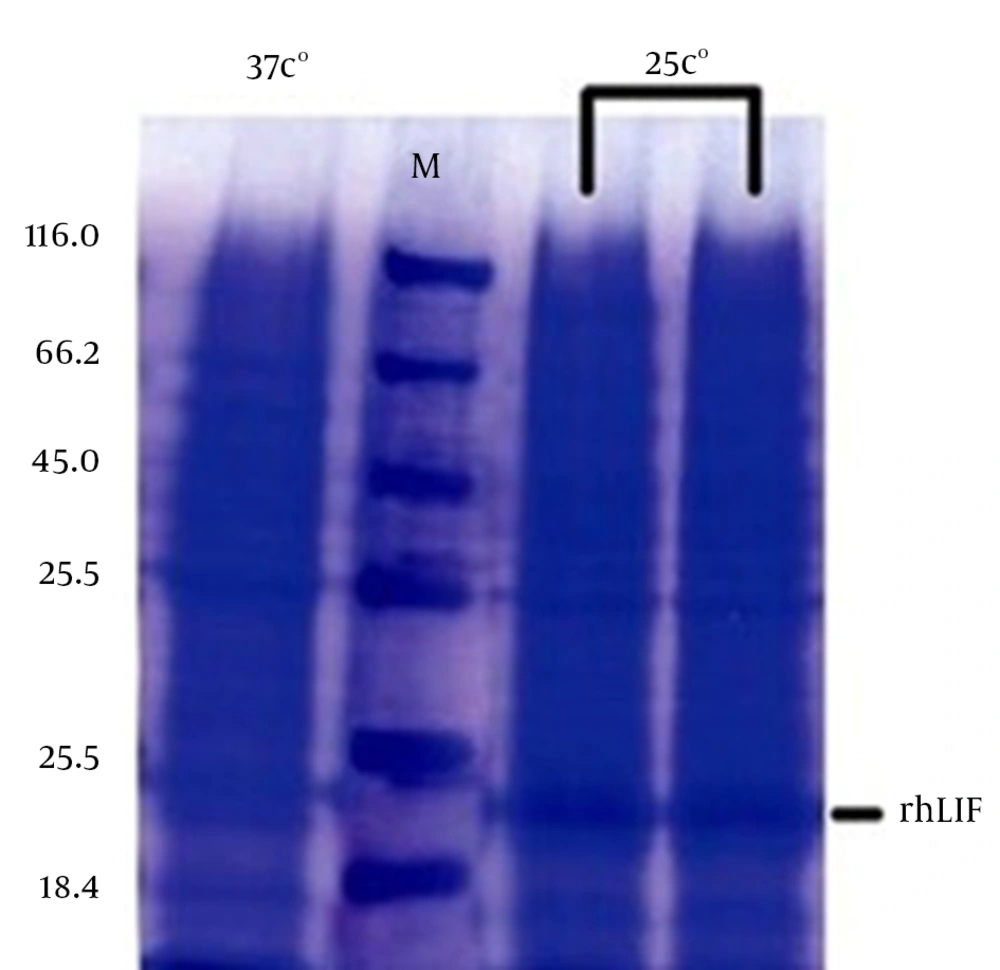
Recombinant protein production by auto induction medium and LB medium in 25ºC was significantly different, auto induction medium result was very low SDS PAGE proved it (Fig. 2).
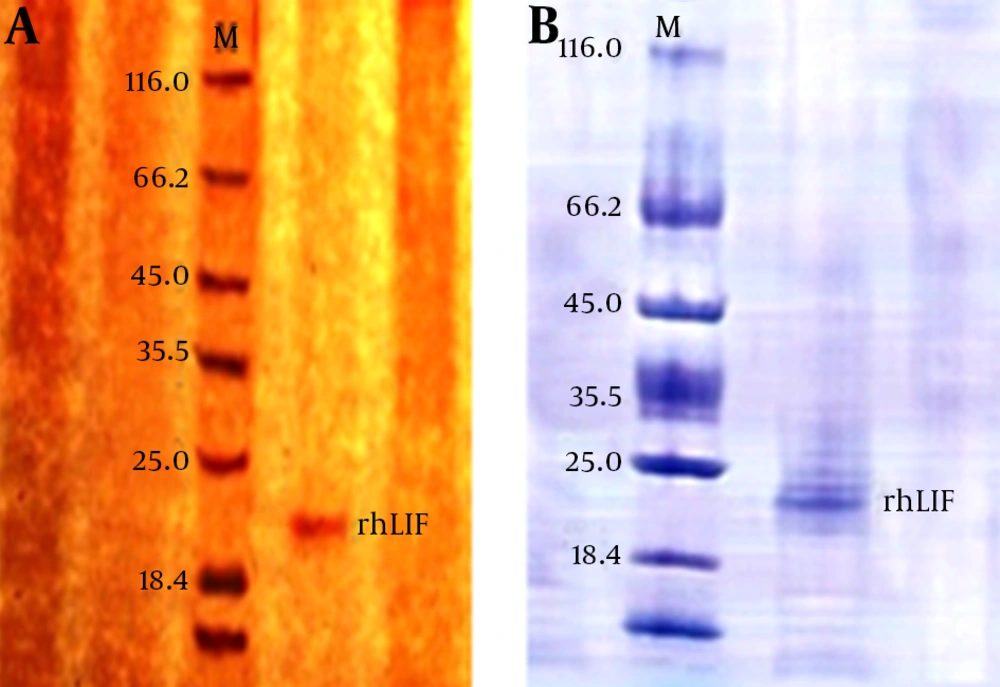
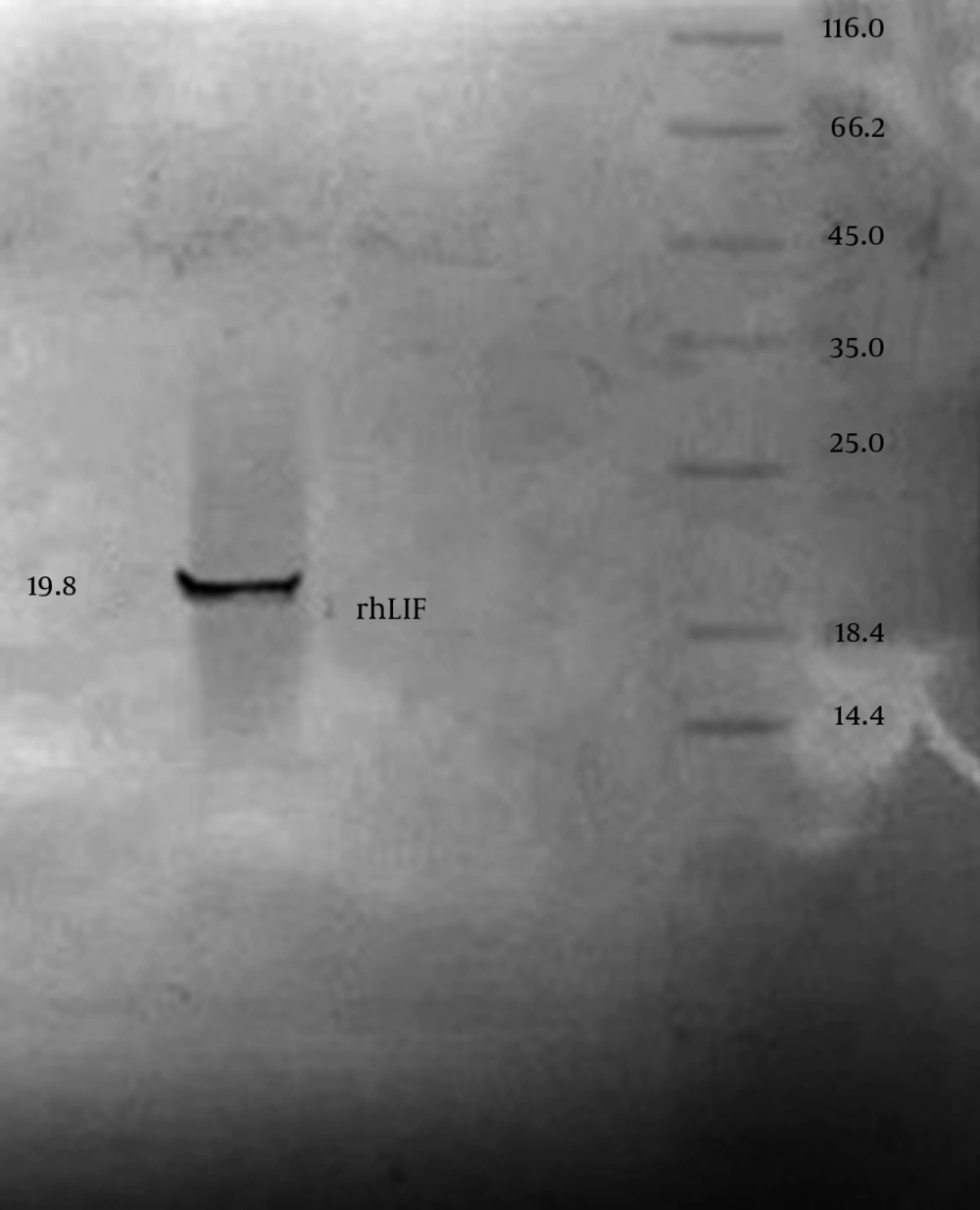
IPTG concentration optimizing and protein purification results: After purification of recombinant protein by chromatography column for analyzing purification accuracy and quality, column result were measured by spectrophotometer at 280 nM and analyzed by SDS PAGE and Western blotting. Measuring results for spectrophotometer is shown in table 1. SDS PAGE gel staining result approved purification of rhLIF, gel staining was done by colloidal Coomasi blue and silver staining (Fig. 3). Western blotting proved accuracy of chromatography purification (Fig. 4).
Discussion
In current study we optimized expression rate of rhLIF for gaining maximum soluble protein for single step chromatography. Our optimization resulted in bacteria culture at 25ºC and induceby 0.4 mmol of IPTG for 3 h which leaded to best outcome. Then we successfully purified recombinant protein in single step purification by affinity chromatography and proved our procedure by SDS-PAGE and Western Blotting.
Leukemia inhibitory factor has remarkable biological and biotechnological role in stem cell culturing. Stem cell increasing utilization in research and medical (cell therapy) makes LIF usage an important issue in economic aspect. According to various effect of this factor on human cell lines, high purity of LIF is notable point that should be considered.
Gough et al. firstly cloned murine leukemia-inhibitory factor (LIF) gene was isolated from a genomic library by using the murine cDNA. LIF gene was introduced into the yeast expression vector YEpsec 1. Yeast cells transformed with the resulting recombinant could be induced with galactose to produce high levels of a factor that induced the differentiation of murine M1 leukemic cells in a manner analogous to murine LIF [19]. Horiuchi et al. cloned chicken LIF in E. coli and studied its effect on maintaining chicken stem cell pluripotent manner maintaining [20]. Tomala et al. cloned LIF by attaching a marker called thioredoxin which leads to gain insoluble recombinant protein production in bacteria cell [21]. Imsoonthornruksa et al. cloned hLIFc DNA into 2 different vectors in order to produce N-terminal His 6-tag and Trx-His 6-tag hLIF fusion proteins in Origami (DE3) E. coli. The His 6-hLIF fusion protein was not as soluble as the Trx-His 6-hLIF fusion protein. One-step immolobilized metal affinity chromatography (IMAC) was done to recover high purity (>95% pure) His 6-hLIF and Trx-His 6-hLIF fusion proteins [18]. Cui and Selwood cloned LIF in brushtail possum which is an eukaryotic host and studied this factor effect in pregnancy maintaining [22]. Abe et al. cloned LIF in zebra fish and indicated that human LIF is orthologous with zebra fish LIF [23]. Song et al. cloned leukemia inhibitory factor (LIF) and assayed its expression in the uterus during embryonic diapause and implantation in the mink (Mustela vison) [24]. By review of limited studies performed understand that production of rhLIF in bacteria makes inclusion body but we can decrease this ratio to the least according to optimization of culturing or utilization of tags such as thioredoxin which is added to recombinant protein to make it insoluble in bacteria cytoplasm to overcome the problem. Our sequence had His-tag for single step purification as it was used before for purifications in other studies too. The problem by tags like thioredoxin is that separating of these tags from recombinant protein need special protease which costs highly and adds extra steps to purification procedure. RhLIF production in E. coli is cost-effective due to the low price of LB medium needed to culture the bacteria in contrast to eukaryotic hosts media and we can gain lots of recombinant protein [25, 26]. Human leukemia inhibitory factor has more suitable response in human and mouse stem cell culturing so achieving recombinant human type of this factor is better that cloning of other animals LIF such the works previously have been done, so we choose human LIF for this propose. In current study all optimizing steps applied to elevate recombinant gene expression to gain high pure rhLIF, Results indicated positive efforts. The only reason can cause reduction in function ability of this factor is glycosylation occurs in human, by the way it seems glycosylation is not necessary for biological activity in vivo nor in vitro, but may affects stability of LIF in vitro or in vivo [1, 3]. Current research experiences maybe useful for same future researches like cancer therapy products and etc. According to restricted access of patients to such products, focusing on such researches would be more important subject.