1. Background
Adipose tissue is an active endocrine organ that produces biological substances called adipocytokins [1]. Adiponectin is one of the most important factors for cardiovascular disease, which is an adipocytokin secreted by adipose tissue [2]. Adiponectin is sensitive to increased insulin and increases nitric oxide production and inhibits ROS (Reactive Oxygen Species) in endothelial cells [3]. Low levels of adiponectin can put a person at risk of diabetes type 2 and coronary artery disease [4]. Changes in adiponectin levels resulted by exercise may have a significant impact on cardiovascular disease associated with obesity and insulin resistance [5-7]. It is revealed that improvements in insulin sensitivity may increase adiponectin levels [8]. Kraemer and Castracane investigated the effects of exercise on adiponectin levels and indicated that the volume of exercise affects the response of adiponectin and the duration and intensity of exercise are considered as important factors in determining the response of adiponectin [9].
Many researchers believe that the increase in adiponectin after prolonged exercise is due to weight loss and improved body composition [10-12]. Dabidi-Roushan et al. showed that a single bout of exhaustive exercise with 65 - 55% HRmax intensity significantly increases adiponectin levels, while during high intensity exercises adiponectin levels insignificantly increased [13]. Mohebbi et al. stated that 12 weeks of aerobic exercise with the intensity of 75 - 80% of Vo2max increases plasma adiponectin in obese middle-aged men [14].
Zeng et al. showed that changes of adiponectin in response to exercise depend on the intensity, duration and frequency of training [15]. While Ferguson et al. showed that a single bout of exercise with 65% HRmax intensity in men and women did not significantly increase the concentration of plasma adiponectin [16]. It was shown that changes in adiponectin concentrations are influenced by nutritional and genetic factors [17]. Also, Punyadeera et al. investigated the effect of 120 minutes exercise with 50% Vo2max intensity on plasma adiponectin in active men and did not observe a significant change [17]. Kobayashi et al. showed that 50 days of walking exercise did not significantly change adiponectin levels in healthy men with normal weight [11]. However, Yatagai et al. concluded that adiponectin levels decreased after 6 weeks of aerobic training in healthy men [18].
Reviewing the literature indicate that the studies which reported no changes in adiponectin after exercise, were more continuous aerobic exercise programs with 50 - 69% Vo2max intensity [11, 19, 20]. Jones et al. stated that 32 weeks of aerobic exercise has no effect on adiponectin [21]. However, a part of this research is devoted to the possible effects of detraining on the levels of plasma adiponectin [4, 22]. Yatagai et al. realized that after 6 weeks of continuous aerobic exercise that led to a significant increase in adiponectin, one week of detraining made adiponectin levels return to its basic levels [18]. While Fatouros et al. showed that after 6 months of strength training with different intensities among the elderly, adiponectin concentrations increased significantly in the groups with high and moderate intensity and after 6 months of detraining adiponectin levels in high-intensity exercise group did not change significantly [20]. The inconsistency in the findings resulted from the effect of exercise on adiponectin, good factor for cardiovascular, is evident. Yet today, aerobic interval training method with more variety and less fatigue is of interest to researchers. In this training method, training time is divided into several stages in each session and some intervals are determined for athletes to rest. This brings up the question that whether by doing these activities in several intervals can make people benefit from protective effects of periodic cardiovascular exercise.
2. Objectives
Therefore, the study aims at investigating the effect of 10 weeks of aerobic interval training and 4 weeks detraining on plasma adiponectin levels in male non-athletes students.
3. Materials and Methods
This is a quasi-experimental study and uses pretest-posttest method and a control group. First, a briefing session was held for male students of Sistan and Baluchestan University who had one Physical Education course, in 3 sessions to give them a complete description about the objectives and subject of the study; 26 male non-athlete students were selected out of 120 students based on simple random sampling and randomly divided into two experimental groups of 13 (with the mean age of 20.1 ± 1.3 years, weight of 74.1 ± 4.4 kg and height of 161.5 ± 1.7 cm) and a control group (age 19.9 ± 1.7 years, weight 75 ± 4.3 kg and height 161.5 ± 5.7 cm). The subjects agreed to participate in the study and filed out medical and health questionnaire and the consent form. During the study, subjects were advised to avoid taking any medication and only use the university’s cafeteria diet. After medical examination, permit was issued by a doctor. In implementation of exercise protocol, safety points were observed to avoid possible damages to participants. In addition, the participants were given the option to leave the study at any time they desire. Students began each practice session with stretching exercises. The experimental group did outdoor aerobic interval training for 10 weeks, three days a week and 55 to 85 HRmax intensity. Intensity and length of the exercises were added according to Table 1 and overload principle. Heart rate of the subjects were calculated using the following equation.
| Weeks of Training | Time of One Training Session, min | 1st Session, min | 2nd Session, min | 3rd Session, min | 4th Session, min | Rest Between Sessions, min | Training Intensity, HRmax% |
|---|---|---|---|---|---|---|---|
| 20 | 5 | 5 | 5 | 5 | 3 | 60 | |
| 21 | 5:15 | 5:15 | 5:15 | 5:15 | 3 | 65 | |
| 22 | 5:30 | 5:30 | 5:30 | 5:30 | 3 | 65 | |
| 23 | 5:45 | 5:45 | 5:45 | 5:45 | 3 | 70 | |
| 24 | 6 | 6 | 6 | 6 | 3 | 70 | |
| 25 | 6:15 | 6:15 | 6:15 | 6:15 | 4 | 75 | |
| 26 | 6:30 | 6:30 | 6:30 | 6:30 | 4 | 75 | |
| 27 | 6:45 | 6:45 | 6:45 | 6:45 | 4 | 80 | |
| 28 | 7 | 7 | 7 | 7 | 4 | 80 | |
| 29 | 7:15 | 7:15 | 7:15 | 7:15 | 4 | 85 |
In order to control the intensity of exercise based on heart rate, an electronic transmitter (heart rate monitor) T, polar 31 model made in Finland was used. Each session ended with cool-down exercises, there were four weeks of detraining after the end of the training program and the subjects of the experimental group avoided any exercises during these 4 weeks. Control group did not have any regular exercise training program during the study and avoided taking any supplements and special diet and continued their normal life.
The selected subjects were invited for anthropometric measurements including height, weight, body mass index, blood pressure, waist to hip ratio (WHR). Their heights were measured three times, in centimeters without shoes and socks on, using a tape measure and the average was recorded as the height of the person. Subjects’ weights were measured three times in kilograms without clothes and shoes and the average was recorded as the weight of the person. Body mass index (BMI), was calculated by dividing weight (kg) to squared height (m2) and WHR was calculated using a tape measure and by dividing waist hip. Blood samples were taken from the subjects in 3 stages of pretest, after 30 session of exercise and after 4 weeks of detraining by laboratory specialists after 12 hours of fasting after 48 hours form the last session, 10 mL of blood from the brachial vein. Blood samples were centrifuged for 15 minutes at 3000 rpm, separated plasma were frozen and stored at -80°C. Plasma adiponectin was measured using ELISA method with Human adiponectin kit manufactured by BOSTER Company of America with a sensitivity of 1 ng/mL, coefficient of variation of the test was less than 0.05. Kolmogorov-Smirnov test is used to evaluate consistency and normality of the data, and repeated measures ANOVA and LSD post hoc test were used to evaluate intragroup differences. The independent t-test was used to evaluate differences between the groups. The statistical significance level of P ≤ 0.05 was used and data were analyzed using statistical software SPSS-17.
4. Results
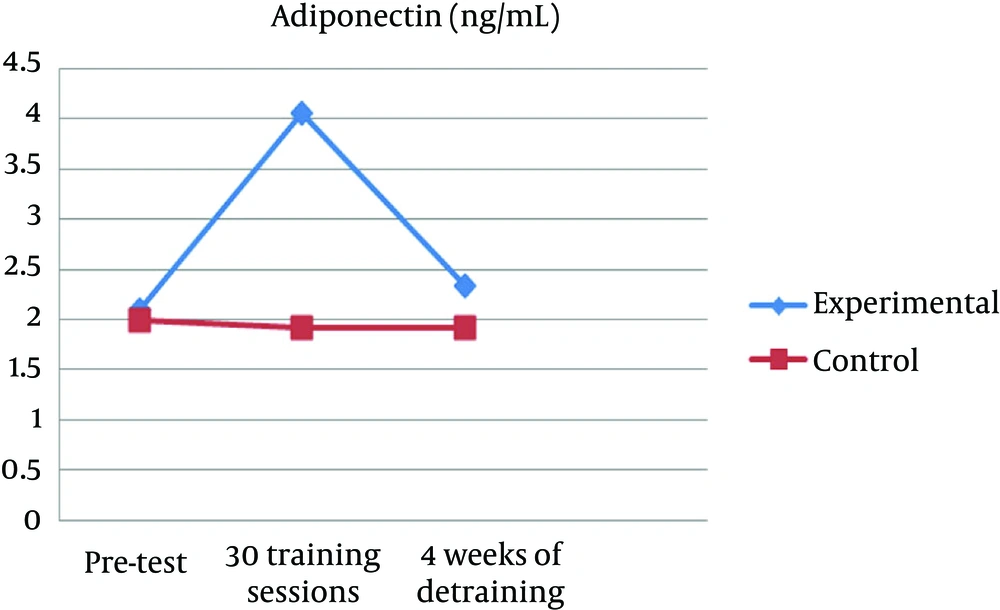
The collected data showed that after 10 weeks of aerobic interval training, a significant reduction in BMI (P = 0.001) and body weight (P = 0.001) (Table 2) and a significant increase in plasma adiponectin levels were observed in the experimental group (P = 0.001), but after 4 weeks of detraining, plasma adiponectin was significantly reduced (P = 0.001). Inter-group comparison between experimental subjects and control group in post-test stage showed a significant increase in plasma adiponectin (P = 0.001) (Figure 1), and significant decrease in BMI (P = 0.022) and body weight (P = 0.038). At the end of the study, there were not significant changes in the values of body weight, BMI and plasma adiponectin (Table 3). At the end of the study, significant differences in plasma adiponectin levels in different stages of experimental groups were observed (Table 4, 5).
| Group | Age, y | Weight, kg | Height, cm | BMI, Kg/m2 | WHR |
|---|---|---|---|---|---|
| 20.1 ± 1.3 | 74.1 ± 4.4 | 167.8 ± 6.2 | 23.7 ± 1.4 | 0.84 ± 0.17 | |
| 19.9 ± 2.1 | 70.5 ± 4.3 | 177.3 ± 0.6 | 22.5 ± 1.2 | 0.79 ± 0.56 |
aValues are presented as mean ± SD.
| Group | Pre-Test | 30 Training Sessions | 4 Weeks of Detraining |
|---|---|---|---|
| Eperimental | 2.09 ± 0.31 | 4.05 ± 0.88 | 2.33 ± 0.35 |
| Control | 2.00 ± 0.33 | 1.92 ± 0.56 | 1.92 ± 0.60 |
| Experimental | 23.75 ± 1.40 | 23.10 ± 1.36 | 24.10 ± 1.09 |
| Control | 22.51 ± 1.16 | 23.04 ± 1.22 | 23.12 ± 1.08 |
| Experimental | 74.10 ± 4.42 | 72.55 ± 4.30 | 73.54 ± 4.30 |
| Control | 70.55 ± 4.32 | 71.05 ± 4.30 | 71.65 ± 5.38 |
aValues are presented as mean ± SD.
bIntragroup significant difference.
| Sum of Squares | Degrees of Freedom | Mean Square | P Value | |
|---|---|---|---|---|
| 39.709 | 2 | 19.584 | 0.001 | |
| 3.152 | 9 | 0.350 |
aMark for statistical significance.
| 30 training sessions | -2.410 | 0.317 | 0.001 |
| 4 weeks of detraining | 0.060 | 0.093 | 0.536 |
| Pre-test | 2.410 | 0.317 | 0.001 |
| 4 weeks of detraining | 2/470 | 0.300 | 0.001 |
| Pre-test | -0.060 | 0.093 | 0.536 |
| 30 training sessions | -2.470 | 0.300 | 0.001 |
aStatistically significance.
5. Discussion
The most important finding of this study is that 10 weeks of aerobic interval training resulted in a significant increase in plasma adiponectin in male non-athlete students, which is confirmed by previous reports indicating that regular physical exercise is associated with higher baseline for adiponectin of good cardiovascular factor. Although there was not any literature on aerobic interval training, the results of this study will be discussed with other methods of physical training on the subject of the research. The results of the present study regarding the significant increase in plasma adiponectin in aerobic interval training group is consistent with findings of Kraemer et al., Kobayashi et al., Mohebbi et al., Zeng et al., and Jurimae et al. [9, 11, 14, 15, 23]. But, research findings of Yatagai et al. are different [18]. Jurimae et al. stated that the greater energy expenditure place the body under more metabolic pressure, and with the increased metabolism, more adiponectin is needed and secreted [23].
In this regard, Ring-Dimitriou et al. studied plasma adiponectin changes in middle-aged suffering from metabolic syndrome during 24 months of exercise and reported increased adiponectin and 15 % increase in cardiovascular-respiratory fitness [24]. Many studies have examined the relation between body composition and the levels of adiponectin and most of the results indicate a negative relationship between body weight [25, 26], body mass index [27, 28], waist circumference [25, 28], fat mass [10, 20, 25] and fat percentage [28] with the adiponectin levels. Thus, one possible reason for the significant increase in plasma adiponectin concentration after 10 weeks of aerobic interval training can be weight loss and improved body composition [29]. In this regard, Goodarzi et al. found that adiponectin concentration was significantly correlated with waist circumference and there is a positive correlation between BMI and WHR in men low adiponectin concentration leads to obesity [30]. Research findings by Mohebi at al. indicate that plasma adiponectin levels in response to aerobic exercise with moderate to high intensity and significant reduction in weight and BMI increased, and there is a relation between the increase in plasma adiponectin levels and decrease in WHR and body fat mass [29]. Some research findings indicated that prolonged exercises had no effect on baseline concentration of plasma adiponectin or these exercises increase the concentration of plasma adiponectin when the exercise vastly reduces the weight or fat mass [9, 15, 31]. This result is clinically important, because due to the effect of adiponectin on the performance of intracellular insulin in skeletal muscle of patients with type 2 diabetes,TRS-1 phosphorylation and the activity of K PI3 decreases. So in case the exercise increases plasma and tissue adiponectin concentration, it will cure the sensitivity to insulin and it is important not only as a therapy, but also as a strategy and a suitable and affordable method in the prevention of diabetes type 2 [32]. It seems that differences in study subjects and limiting factors (such as nutrition, disease, etc.) are the reason for difference between this research and the present study. On the other hand, there are studies that despite the effect of exercise on losing weight and body fat, plasma adiponectin concentration levels remain unchanged [33]. Also, Hamedinia et al. showed that after 13 weeks of aerobic exercise in healthy non-athlete men did not observe any change in adiponectin concentration, therefore it is necessary to keep this in mind in interpreting the relationship between weight loss and changes in. Therefore it is necessary to interpret the relationship between weight loss and changes the subject considered adiponectin [34]. In this regard, Khedri et al. found that 8 weeks of aerobic exercise did not significantly change plasma adiponectin concentration [35]. Perhaps the subjects in the current study are the reason for the difference in the results of the present study. Ahmadizad et al. examined the effect of aerobic exercise against resistance on adiponectin and concluded that there is a negative significant relationship between adiponectin levels and percentage of body fat compared to WHR and BMI [36]. Coker et al. investigated the effect of exercise intensity (50% vs. 70% peak oxygen consumption) abdominal fat and adiponectin levels in the elderly. But no change was observed in plasma adiponectin [37]. The contradiction of the results of the study in plasma adiponectin compared to other studies may be due to factors such as differences in study groups during exercise, exercise intensity and type of exercises. Adiponectin gene expression is regulated by the size of adipocytes, as the size of adipocytes increases, adiponectin gene expression increases too. With increasing obesity, the size of adipocytes increases and adiponectin gene expression decreases after weight loss, adipocytes secrete more adiponectin. Since adipocytes released TNF-α and IL-1ß inflammatory mediators known as inflammatory markers, any decrease in the release of inflammatory mediators from adipose tissue is associated with reduced inflammation and fatigue [12, 14]. In this study significant changes in body weight and BMI group practice may be associated with the reduction in body fat mass which, in turn, reduces the production of inflammatory markers and fatigue which is the mechanism for the increase in plasma adiponectin in the subjects. Furthermore, reports show, regular aerobic exercise reduces sympathetic stimulation and increased anti-inflammatory cytokine, inhibits release of inflammatory mediators TNF-α and IL-ß from adipose tissue and subsequently reduces inflammation and fatigue [13, 38]. It is known that during aerobic exercise, endocrine system increases oxidation of lipids (lipolysis) by increasing epinephrine, norepinephrine, growth hormone, and cortisol hormones and by increase in calls and using free fatty acids to produce energy during the workout, provides the required energy for muscles and reduces body fat mass [38]. Therefore, in this study, the aerobic interval training was probably accompanied with increase in lipid oxidation and decrease in body fat mass which is a mechanism to increase plasma adiponectin. On the other hand, the persistence of changes in adiponectin after exercise is an issue that has been largely ignored. In the present study a significant decrease in adiponectin levels was observed after 4 weeks of detraining. There are very few studies in this area. In this regard, Yatagai et al. realized that after 6 weeks of continuous aerobic exercise that led to a significant increase in adiponectin, one week of detraining made adiponectin return to its basic levels [18]. So, probably the similarity between the results of this research and the present study are in the type of subjects and training method which in this research and the present study lead to decrease in plasma adiponectin. It can be pointed out that after detraining, adaptations resulting from exercise in subjects disappear and risk of cardiovascular disease threatens them. while Fatouros et al. showed that after 6 months of strength training with different intensities (low, medium, high) among the elderly, adiponectin concentrations increased significantly in the groups with high and moderate intensity and after 6 months of detraining adiponectin levels in high-intensity exercise group did not change significantly. They found that after 6 months of detraining, there were no significant changes in the weights of high intensity exercise group and there was no relation between adiponectin and body weight changes [20]. Ziccardi et al. found that imbalance between received and expended energy which results from detraining leads to weight gain and obesity in people, threatens healthiness of cardiovascular system and can cause cardiovascular disease [38]. The present study had limitations, such as long duration of training period in which the researches tried to control subject’s diet to some extent and suggested them to comply with the diet plan of the university’s cafeteria and follow the specified diet as much as possible and avoid doing any regular exercise apart from the training protocol.
It is generally suggested that those who cannot run for a long time, can benefit from its cardiovascular protective effects by doing it in several consecutive turns. According to the results, it can be stated that aerobic interval training as an effective and beneficial training method with more variety and less fatigue, with a significant increase in plasma adiponectin, cardiovascular protective factor, has a preventive effect on cardiovascular disease; while with cessation of exercise and detraining, the desired adaptations disappear and cardiovascular protective factor decreases and body is exposed to cardiovascular risk factors.