1. Background
Now a day, nanoparticles is used in most industries because of their properties. The important features of iron oxide nanoparticles such as fast effect, high magnetic property and small size are caused that iron oxide nanoparticles will be used as a contrast agent in MRI (Magnetic Resonance Imaging) and NMRI (Nuclear Magnetic Resonance Imaging) and the diagnosis of cancers.
It has reported that some nanoparticles make reactive oxygen (Reactive oxygen species: ROS) [1] and cause toxicity in lab environment [1]. Very small size, large surface area and reactiveness of nanoparticles will expose people to serious dangers potentially. Nanoparticles can easily cross the cell membrane [2] and block blood-testicular [3]. The toxicity of iron oxide nanoparticles by creating changes in pH and serum concentration has been reported [4]. The studies which were performed on mice about Tio2 nanoparticles showed that nanoparticles can be seen in Leydig cells, Sertoli and spermatids [5]. In another report the effect of black carbon nanoparticles on the performance of male genital system were investigated, decreased daily sperm production. Testicular morphology changes especially Leydig cells and the reduction of testosterone density [6]. The effects of nanoparticles in various cells have decreased mitochondrial activity [7]. However, in spite of the importance of reproductive system a few studies have done on the iron oxide nanoparticles effects on sexual cells.
2. Objectives
The present investigation was set to evaluate the effect of iron oxide nanoparticles on male sexual cells and sexual hormones.
3. Materials and Methods
In this experiment study, iron oxide nanoparticles with a diameter of 30 nm were purchased from the Nano Technology Research Center of Yazd Payame Noor University. First, 300 mL (0.2 M) of iron nitrate Fe(NO3)3. 9H2O (Aldrich 98%) was used as a precursor solution, and was gelated by using 800 mL of mono hydrated citric acid (Aldrich 98%) solution (0.05 to 0.3 M) as ligands with a molar ratio of 1:1 were distilled water as the solvent. The iron solution was added to the citric acid solution drop wise with vigorous stirring. The solution was then heated to a temperature of 80°C, while maintaining vigorous stirring until the gel was formed and the contained water was evaporated. The dried gel was annealed at temperatures ranging from 200 - 400°C, typically yielding Fe2O3 ranging in size from 20 - 30 nm. Then, they were deyonized and were changed to a solution and were prepared in different concentration 5, 10, 20, 40 mg/kg. There is not any coating to review the features of this nanoparticle dispersion was performed crystal X-ray diffractometer (XRD) for the confirmed the crystal structure and for the average size of the particles. However for nanoparticle size composite photo electron microscope (Transmission electron microscopy: TEM) was used to set both of these analysis showed that the synthesis of nanoparticles in diameter of about 20 ± 5 nm found with using the Scherrer formula [1].
In this study, 57 mice (were purchased from Pastor Institute, Tehran, Iran) were used. Mice randomly divided into 5 groups (N = 15): control group and 4 experimental groups that received iron oxide nanoparticles at the dose levels of 5, 10, 20, 40 mg/kg intraperitaneally (i.p.) for 2 weeks [8]. This research was approved with ethics committee of Payame Noor University. The mice were kept under standard conditions [8, 9].
One week after the last injection (after passing the spermatogenesis period) the mice were anesthetized by chloroform, then the abdomen of animals was opened and reproductive organs (testis and epididymis) were removed.
Epididymises were dissected out, immediately minced in 5 mL of physiological saline and then incubated at 37°C for 30 minutes to allow sperms to leave the epydidimal tubules [8, 9]. The cauda epididymis sperm were determined using the standard hemocytometris method.
For the histological examination, small pieces of testis were fixed in 10% formalin, dehydrated and then sectioned. Sections were then stained with Hematoxylin and Eosin and examined under light microscope [8].
Statistical analysis was performed with SPSS-13. All parameters were analyzed by one way ANOVA and the obtained data were expressed as Mean ± SD. For parameters with significant differences among groups, multiple comparison tests were carried out (P < 0.05) [8, 9].
4. Results
TEM and XRD analysis showed that the synthesis of nanoparticles has a diameter of about 20 ± 5 nm.
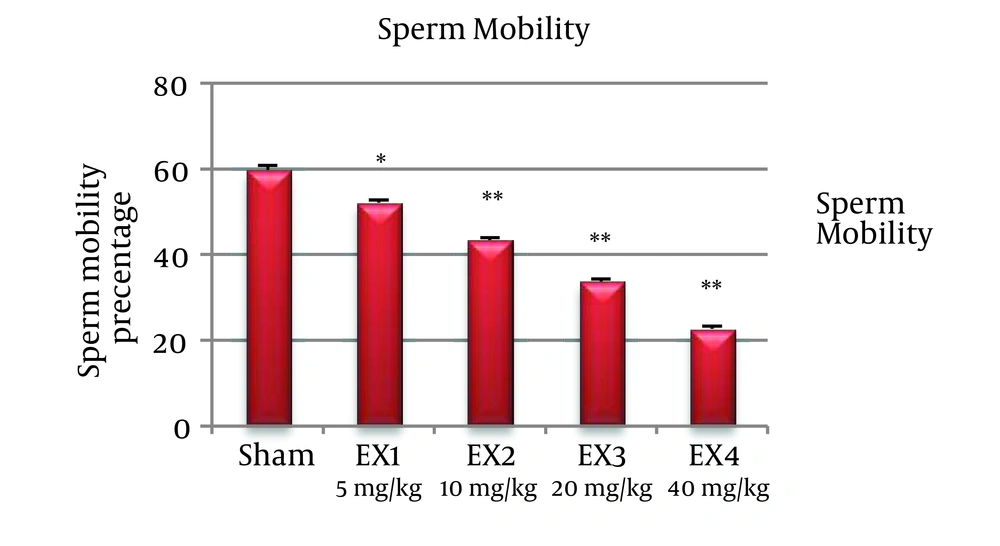
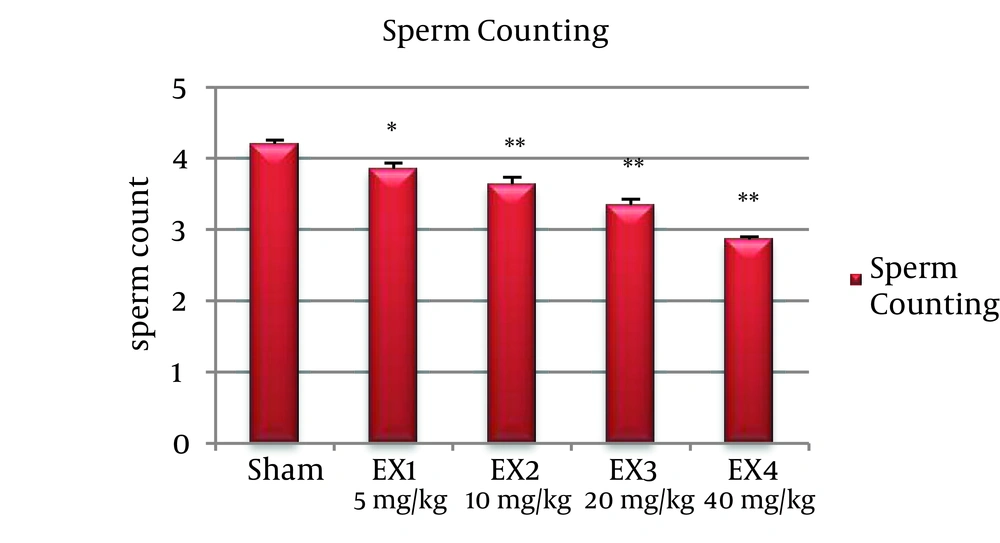
The number of sperms was significantly reduced in all experimental groups (P < 0.001). Percentage of mobile sperms, decreased in all experimental groups significantly (P < 0.001) (Figures 1 and 2).
Investigation of seminiferous tubules showed reduction of Spermatocytes and spermatids detachment of Spermatogonia and Spermatocytes from the wall of the tubule and the release of sperm precursor cells into the middle of the tubule.
5. Discussion
The investigation of sperm mobility in the epydidimal area shows that the percentage of sperm mobility in the mice in experimental group in relation to control group has had a meaningful decrease, that it can be said the reason is related to the effect of iron oxide nanoparticles on mitochondrial action [5].
The researches which have done on the effect of nanoparticles on mitochondrial action in different cells have shown the reduction of mitochondrial action in all cases [5]. The studies which performed by Braydich-Stolle et al. on the line 4 - 18 cells showed that nanoparticles such as iron oxide and silver can cross the membrane of sperm and they can connect to mitochondria and acrosome of sperm [5]. On the other hand, sperm mobility was happened in epididymis the investigation shows that nanoparticles can effect on epididymis and cause the inflammation of epididymis that it can reduce sperm mobility. Also nanoparticles in cells cause the increasing of ROS that this can decrease the mobility of the cells by damaging to the flagella structure of the sperms [10].
Metal nanoparticles increase ROS such as super oxide which may cause the inhibit of RNA polymerase [10], the oxidation of molecules such as protein and even DNA and in this way they cause the decrease of LH, testosterone and Leydig cells [11].
Histopathological examination and statistical analysis shows that the number of sperms in all experimental groups has been decrease. Iron oxide nanoparticles can damage the DNA of leyding cells; the apoptosis of these cells and at last cause their death [12].
This study was showed iron oxide nanoparticles have negative effects on sperms and it is suggested that the effect of iron oxide nanoparticles on Leydig cells occurs at the molecular level.