1. Background
Osteoarthritis (OA) is the most common rheumatic disorder with increasing prevalence with advancing age [1]. This disorder is a progressive degenerative joint disease that has a major impact on joint function and quality of life. Today, there is no curative treatment for OA so, pharmacologic and non-pharmacologic modalities are used to improve pain and disability [2].
Pharmacological management of OA has targeted symptoms of the disease not the underlying cause. The nonsteroidal anti-inflammatory drug analgesics (NSAIDs), selective cyclooxygenase 2 (COX-2) inhibitors and intra-articular therapies like glucocorticoid and hyaluronan injections have been used as a pain reliever but they are reported to be associated with some adverse effects [2]. Patients with chronic painful disorders such as OA often tend to receive alternative therapy such as herbal medicine. The efficacy and safety of most alternative treatments have not been established by controlled trials.
There is some evidence about nutraceuticals in the management of OA but there is not sufficient reliable evidence regarding safety or effectiveness [2-8]. The beneficial effects of medicinal plants such as ginger and Elaeagnus angustifolia have been reported [3-5, 9-12]. The ginger herb is extensively used in the tropical areas and E. angustifolia was suggested to have had analgesic and anti-inflammatory effects [3]. Herbal anti-inflammatory medicines provide a broad spectrum mechanism of action and they interact to various extents with the inflammatory cascade, elastase or hyaluronidase inhibition, antioxidative effectiveness and other still unidentified effects that may contribute to the overall analgesic and joint protective effects. But experimental data also indicate interaction with cytokine production [6]. There are some trials about the efficacy of ginger extract [4, 5, 9] or E. angustifolia in [3] in OA but we tried to evaluate the efficacy and safety of combining ginger and E. angustifolia extracts in knee osteoarthritis.
2. Objectives
This is a study for evaluation of the efficacy and safety of Elaeagnus angustifolia and ginger extracts in patients with osteoarthritis of the knee.
3. Patients and Methods
3.1. Subjects
This randomized double-blind clinical trial study carried out on 80 outpatients with knee OA. The selected patients were 45 - 60 years old (the mean age was 52.0 ± 4.8 years) with moderate to severe knee pain (VAS ≥ 4) visited in a rheumatology clinic in Sari, Iran as outpatients. The simple size was estimated according to some similar studies [3, 5, 13]. All patients had complaints of clinical dysfunction and pain due to knee OA with pain on movement of more than 4 cm on a 10 cm VAS (mean 6.7 ± 1.8) at first visit. The classic criteria method for OA of the knee is based upon the presence of knee pain plus at least 3 of the following 6 clinical characteristics: greater than 50 years of age, morning stiffness for less than 30 minutes, crepitus on active motion of the knee, bony tenderness, bony enlargement and no palpable warmth [14]. Patients were excluded if they were pregnant or lactating, if their disorder was secondary to inflammatory arthritis, or with uncontrolled hypertension (blood pressure ˃ 140/90), heart failure (class 3 or 4), renal failure (serum creatinine ˃ 1.5), liver dysfunction (liver enzyme ˃ 1.5 times more than normal), raised ESR (according age and sex), history of peptic disease or bronchospasm, and cerebral vascular accident or myocardial infarction in past year. The study was approved by the Ethics Committee of Mazandaran University of Medical Sciences and was recorded in IRCT (IRCT code: IRCT138812121828N3).
3.2. Plant Material
E. angustifolia (whole seeds) and ginger (rhizomes) were authenticated by Dr. B. Eslami (assistant professor of plant systematic) and the voucher specimens were deposited (Nos. 1191 and 1193) in the Sari School of Pharmacy herbarium. The dry materials were coarsely ground, obtaining 1-2 mm particles and then extracted separately by water for 24 hours at room temperature. The extracts were then separated from the sample residues by filtration through Whatman No.1 filter paper. Extraction was repeated three times. The resulting extracts were concentrated over a rotary vacuum at 45°C until a crude solid extracts were obtained which then were freeze-dried for complete solvents removal (yields 21.7 and 5.7%, respectively). For standardization, the total phenol and flavonoids of extracts were determined.
3.3. Determination of Total Phenol and Flavonoid Contents
Total phenolic compound contents were determined by the Folin-Ciocalteu method [15]. The extract samples (0.5 mL) were mixed with 2.5 mL of 0.2 N Folin-Ciocalteu reagents for 5 minutes and 2.0 mL of 75 g/L sodium carbonate were then added. The absorbance of mixture was measured at 760 nm after 2 hours of incubation at room temperature. The standard curve was prepared by 0, 50, 100, 150, 200 and 250 mg/mL solutions of gallic acid in methanol: water (50:50, v/v). Total phenol values are expressed in terms of gallic acid equivalent (GAE), mg/g of extract), which is a common reference compound. The total flavonoid content was measured by a colorimetric aluminum chloride method [15]. Briefly, 0.5 mL solution of extract in methanol was mixed with 1.5 m: of methanol, 0.1 mL of 10% aluminum chloride, 0.1 mL of 1 m potassium acetate, and 2.8 mL of distilled water and left at room temperature for 30 minutes. The absorbance of the reaction mixture was measured at 415. The total flavonoid contents were calculated as quercetin from a calibration curve. The calibration curve was prepared by preparing quercetin solutions at concentrations 12.5 to 100 mg/mL in methanol. The total phenol contents with reference to the standard curve (y = 0.0054x + 0.0628, r2 = 0.987), were 73.4 ± 2.1 and 201.7 ± 4.3 mg GAE/g of extract for E. angustifolia seeds and ginger rhizomes extracts, respectively. The total flavonoid contents were 17.6 ± 0.6 and 18.9 ± 0.5 mg quercetin equivalent (QE)/g of extract for E. angustifolia and ginger extracts, respectively, with reference to the standard curve (y = 0.0063x, r2 = 0.999).
The dried extracts were powdered and mixed with starch powder and then were encapsulated. Each capsule contained 100 mg extracts (i.e. 13.75 mg total phenols and 1.85 mg flavonoid). Starch powder was used as placebo. Final formulations for clinical trial were controlled microbiologically.
3.4. Design
This is a randomized clinical trial study performed on 80 outpatients from a rheumatology clinic in Sari, Iran, who were suffering from knee osteoarthritis. After obtaining signed informed consent, the patients were randomized to receive drug or placebo by drug code group 1 (32 patients), who received the 200 mg extract of E. angustifolia and ginger and group 2 (29 patients) who received placebo. Both patients and investigators were blinded to treatment assignment.
Before the intervention, knee radiography, serum creatinine, liver enzymes, ESR, blood pressure were done. Analgesics (NSAIDs and acetaminophen) use was allowed during the study and their consumption in the past week was recorded. Injections in joints were not allowed during the study. The drug and placebo was administered 2 times per day for 2 months by a trained medical student and the patients were evaluated for capsule consumption by history and controlling of drug boxes 2 weeks later. Then the drug or placebo was given to patients for the continuity of the study.
3.5. Blinding
Both the drug and placebo were indistinguishable from the original, and as long as it was intact, the capsule did not convey any smell or taste. The packages were similar and the patients and physicians were blind to drug or placebo. By the end of the double blind trial at week 8 the code was broken and data analysis was done.
3.6. Assessment of Efficacy
The assessment of efficacy was done for pain severity by visual analogue scale (VAS), patients and physician global assessment of efficacy (according to a 4-scale scoring system: 0 for inadequate, 1 for moderate, 2 for good and 3 for excellent), and the amount of analgesic use in the past week by an observer blinded to treatment allocation. A responder was defined by a reduction in pain of ≥ 2 cm on a visual analog scale [4].
3.7. Assessment of Safety
For the assessment of safety, the adverse events such as dyspepsia and headache were recorded. At entry and end treatment period, the blood samples were obtained to measure the serum creatinine and liver enzymes. Statistics: statistical analysis was done by the SPSS-12 using χ2 test, Independent t-test and Wilcoxon Signed-rank test for comparisons of mean values of patients at baseline and after the intervention. The level of significance was assumed 0.05.
4. Results
The study was performed during 2011 - 2012. Eighty patients with knee osteoarthritis were enrolled in the study, 40 patients in the drug group and 40 patients in the placebo group. Nineteen patients were excluded, 3 patients in the placebo group and 2 patients in the drug group refused participation and 8 and 6 patients dropped out, respectively because of drug complication or inefficacy, so the statistical analysis was done on 61 remaining patients. Seventy eight (97.5%) patients were females and 77 (96.3%) were housewives. The mean of BMI was 30.0 ± 4.5 kg/m2. Most of the patients had previously tried NSAIDs and an entry 53 (66.2%) of patients were receiving analgesics. The demographic and baseline characteristics of the patients were shown in Table 1.
| Drug Group | Placebo Group | P Value | |
|---|---|---|---|
| 50.1 ± 4.5 | 53.3 ± 4.8 | 0.03 | |
| 100 | 95 | 0.49 | |
| 30.9 ± 4.4 | 29.1 ± 4.5 | 0.12 | |
| 120.5 ± 10.4 | 119.7 ± 8.9 | 0.73 | |
| 6.9 ± 2.1 | 6.6 ± 1.5 | 0.47 | |
| 0.30 | |||
| None | 3 (8.3) | 1 (2.6) | |
| Mild | 21 (58.3) | 28 (73.7) | |
| Moderate | 8 (22.2) | 8 (21.1) | |
| Severe | 4 (11.1) | 1 (2.6) | |
| 17.3 ± 11.9 | 21.0 ± 9.8 | 0.13 | |
| 0.87 ± 0.19 | 0.85 ± 0.16 | 0.58 | |
| 21.6 ± 8.5 | 20.9 ± 4.5 | 0.63 | |
| 20.9 ± 4.5 | 20.3 ± 7.4 | 0.61 | |
| 4.2 ± 4.4 | 3.8 ± 4.9 | 0.70 |
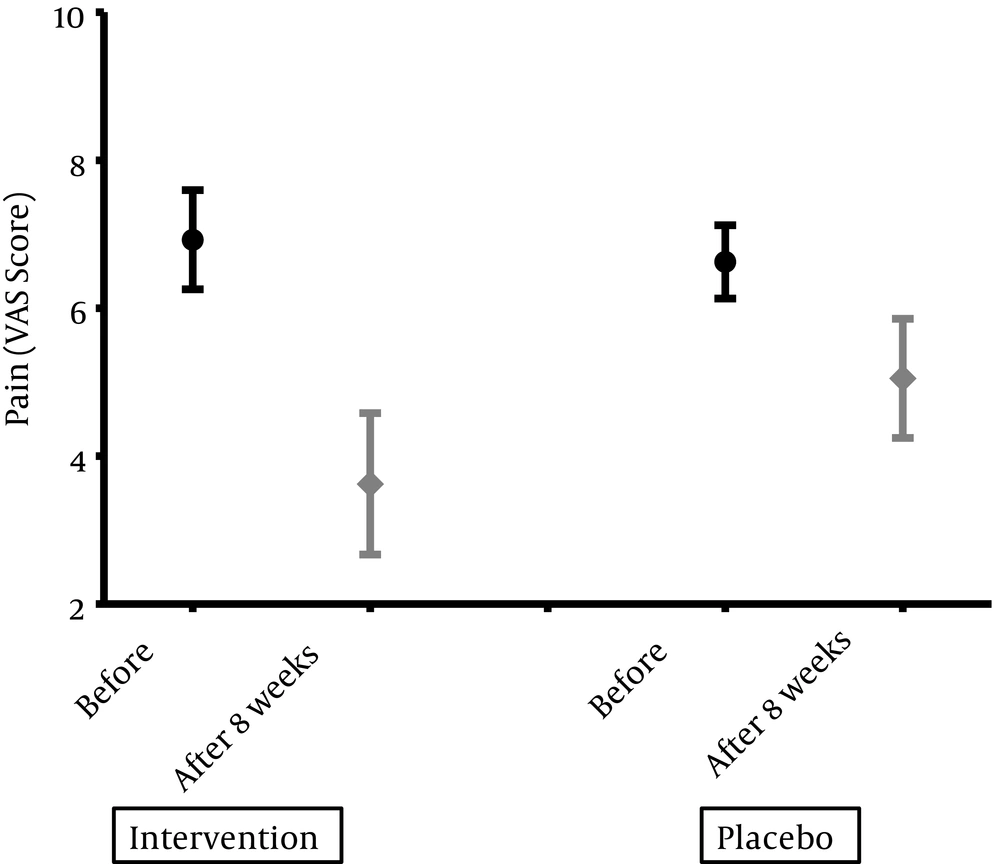
Eight weeks after the intervention, two groups were evaluated for clinical and biochemistry variables. To compare at baseline values, the patients in drug group had more improvement in pain intensity according to VAS (P = 0.02) and the physician evaluation according to a 4- scale scoring (P = 0.03). Changes in pain and disability indexes have been shown in Figure 1 and Table 2. Analgesic consumption in drug group changed from 4.2 ± 4.4 to 3.8 ± 4.8 and in placebo from 3.8 ± 4.9 to 3.1 ± 5.28.
| Drug Group | Placebo Group | P Value | |
|---|---|---|---|
| 3.62 ± 1.5 | 5.05 ± 2.1 | 0.02 | |
| 0.07 | |||
| Not enough | 4 (12.5) | 8 (27.6) | |
| Moderate | 8 (25) | 12 (41.4) | |
| Good | 12 (37.5) | 7 (24.1) | |
| Excellent | 8 (25) | 2 (6.9) | |
| 0.02 | |||
| Not enough | 3 (9.4) | 9 (31.0) | |
| Moderate | 11 (34.4) | 11 (37.9) | |
| Good | 12 (37.5) | 9 (31.0) | |
| Excellent | 6 (18.8) | 0 | |
| 19.35 ± 12.6 | 20.93 ± 14.0 | 0.24 | |
| 0.86 ± 0.17 | 0.87 ± 0.13 | 0.66 | |
| 21.6 ± 8.1 | 21.6 ± 6.3 | 0.96 | |
| 21.0 ± 11.7 | 19.36 ± 7.8 | 0.18 | |
| 3.8 ± 4.85 | 3.1 ± 5.28 | 0.87 |
Thirty patients in drug group and 28 patients in placebo group completed the study without any complications. The patients in both groups had experienced some side effects.
Totally 8 complications in drug users and 9 in placebo group were recorded (21.1% vs. 24.3%). In drug group, 3 patients experienced dyspepsia, 2 headache, 1 raised blood pressure, 1 fatigue and 1 rash. In placebo group, 4 participants had dyspepsia, 1 headache, 1 oral ulcer, 1 itching, 1 raised blood pressure and 1 palpitation.
Because of the dropped outs (8 patients in drug group and 11 in placebo group), the analysis of baseline observation was done and it was showed a significant decrease in pain severity according to VAS (P = 0.03).
5. Discussion
This study demonstrates a significant change on pain intensity and physician evaluation of the patients with knee OA after 8 weeks of using 200 mg combined E. angustifolia and ginger extract more effective than placebo. Pain severity according to VAS was reduced as 3.45 ± 2.0 and 1.74 ± 2.2 in drug and placebo groups, respectively and the patient’s self-evaluation had trend to reduce significantly. During the study, there were some subtle but not biochemical side effects and there was not any difference between the drug and the placebo. In our subjects, two thirds of the patients were regular analgesic users.
Although the use of NSAIDs in OA is controversial and is frequently limited by side effects, but many patients use them for relieve pain with or without the physician administering them. The ginger herb is extensively used in the tropical areas for cooking or to cure some various illnesses several thousand years ago [5]. E. angustifolia grows in Asia and Europe and was suggested to have had analgesic and anti-inflammatory effects [3]. The major constituents of ginger include volatile oils, oleoresin (gingerol), linoleic acid and trace elements such as magnesium, phosphorus, and potassium [2]. In vitro studies suggest that [6], gingerol is a potent inhibitor of NO synthesis and also an effective protector against peroxynitrite-mediated damage [16, 17] and it is effective in inhibiting the production of PGE2, TNFα, and COX-2 expression in human synoviocytes by regulating NF-κB activation and degradation of its inhibitor IkB-α [18].
It has been shown that E. angustifolia extract contains flavonoid components, terpenoids and cardiac glycosides. Flavonoids have muscle relaxant activity, anti-nociceptive, anti-inflammatory effects [19, 20], interact with prostaglandins and arachidonic acid metabolites [21] and have antioxidant effects [21].
There are some trials about the efficacy of ginger extract [4, 5, 9] or E. angustifolia in [3] in OA. A randomized, placebo-controlled, cross-over study comparing 170 mg ginger extracts and 400 mg ibuprofen or placebo for 3 weeks was performed on 75 patients with OA of the hip or knee. A ranking of efficacy of the three treatment periods: Ibuprofen > ginger extract > placebo was found for visual analogue scale of pain and the Lequesne-index. The study of Bliddal et al. showed significant improvement in symptoms for both groups before crossover; however, at the study’s end there was no difference between ginger and placebo [5]. Short term duration of treatment, dose of ginger extract, former use of ibuprofen in some groups (no further wash-out periods were used between the three treatment periods) and non-pharmacological treatments may influenced the study, but in present study, we tried to a conduct a randomized clinical trial with longer period.
A randomized, double-blind, placebo-controlled trial study studied the effects of ginger in the treatment of knee OA in 261 patients. The patients received ginger extract or placebo twice daily, with acetaminophen allowed as rescue medication. The primary endpoint of the study was pain on standing after 6 weeks. In the ginger extract group 63% versus 50% in the placebo group showed improvement in knee pain on standing and after walking 50 feet and reduction in the Western Ontario and McMaster Universities osteoarthritis composite index. Change in global status and reduction in intake of rescue medication were numerically greater in the ginger extract group. The study failed to show improvement in quality of life [4]. It had a similar design of study, but we had a longer period of study.
In another study, efficacy of 30 mg/day ginger extract for one month was similar to 400 mg ibuprofen and superior to placebo. The clinical assessments included VAS for pain, gelling pain, joint swelling measurement, and joint motion slope measurement. The improvement of symptoms was superior in the ginger extract and ibuprofen groups than the placebo group. However, there was no significant difference in VAS and gelling pain scores between the ginger extract and the ibuprofen groups. The authors concluded that one month duration of therapy with only one dose of ginger extract application might not have been adequate for all the effects of ginger extract to be detected [9].
A placebo controlled double blind study was done on E. angustifolia in patients with knee osteoarthritis, pain according VAS and function as improvement in Lequesne’s Pain Function Index, global assessment of efficacy and tolerability by patient and physician, showed decreased pain and function more than acetaminophen and placebo [3].
In present study, we tried to efficacy and safety of combination of two probably herbal medicines in knee OA for 8 weeks. The patients in both groups experienced some improvement in pain intensity and this might be because of more care. Of all patients, 28 (87.5%) in drug group and 21 (72.4%) in placebo group experienced some improvements in pain and disability, and 29 (90.6%) and 18 (69%) in physician evaluation of patients respectively. Liver enzyme and serum creatinine were stable in two groups.
There are some limitations in this study. The intervention period was 8 weeks and it might not be enough for the evaluation of long term efficacy or safety. Radiologic change needed long term duration and this study could not evaluate it, so we did not repeat knee X-ray. The pain reduced significantly but analgesic consumption did not change. This was similar to Altman and Marcussen study [4] and may be because of study duration or drug dose. Patients in drug group were younger than placebo group but clinically it may not important because they were similar in other symptoms or signs and covariance analysis according to age showed that an improvement in drug group was significantly consistent.
Our patients had a moderate to severe pain and not reducing analgesic consumption may be because of severity of pain before the intervention. We suggest further studies with different doses and durations of intervention of combined E. angustifolia and ginger extract. These studies may suggest that patients should change consuming NSAIDs to more harmless substances.