1. Context
Burton in 1882 was found an analogue of valeric acid with two propyl groups called valproic acid (VPA) [1]. Indeed VPA is a carboxylic acid, which is a clear liquid at ambient temperature. Eighty years later the anti-epileptic properties of this drug have been recognized and then again five years later it has been approved as an anti-epileptic drug (AED). In fact it was accepted by the US Food and Drug Administration in 1978 for management of some kind of epileptic seizures such as complex partial seizures and simple and complex absence seizures. An equimolar mixture of VPA and sodium valproate called divalproate, has been accessible in the United States since at 1983. The other brand names are Depakene, Depakote, Depakote ER. Its’ international brand name called also Depakine. Two forms of drugs have extra FDA approvals. The extended-release form of drug (Depakote ER) is designated for migraine headache prophylaxis and the delayed-release product (Depakote) carries an indication for the short term management of acute manic episodes associated with bipolar disorder [1-4].
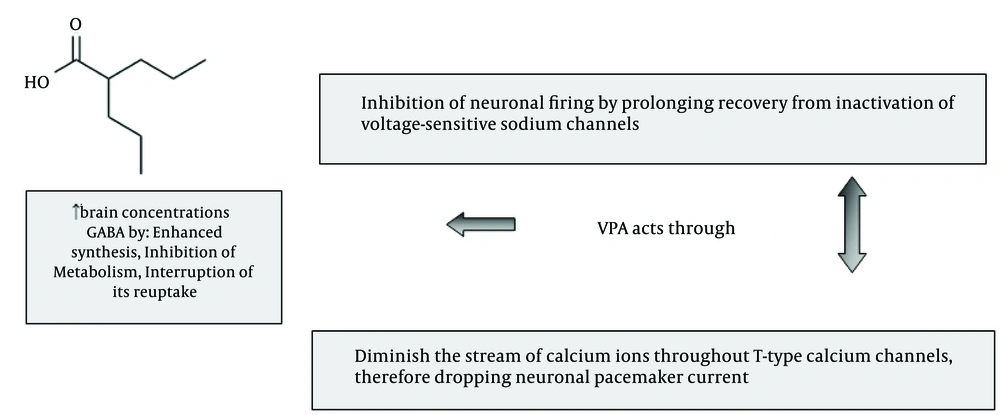
As shown in Figure 1, VPA has a different mechanism of actions. In bipolar disorder it effect by inhibiting gamma-aminobutyric acid (GABA) transaminase and then increases the amount of GABA within brain. However, several other mechanisms of action have been proposed for this AED in recent years. The drug could be categorized as a broad-spectrum AED, because it suppress both sodium and calcium channels. It is a histone deacetylase inhibitor. Sodium valproate possibly could affect sodium channels and potentiate GABA receptors. It also increases the formation of GABA [5]. It is well known that VPA belongs to group of drugs with narrow therapeutic window and as prescription of VPA in Iranian epileptic patients continues to increase [4-6], an approach adjoining clinical pharmacotherapy guideline to control seizure attack was of interest that reviewed.
2. Evidence Acquisition
Data of this review were collected from the authors clinical experiences’ related to pharmacological, biochemical inter and intra individual management of antiepileptic drugs in patients with epilepsy [5-7]. Directory of Open Access Journals (DOAJ), Google Scholar, PubMed (NLM), LISTA (EBSCO), Web of Science were searched. The key words were relevant to Valproic acid, Pharmacokinetics, Epilepsy and Iran. A total of approximately 1200 articles were found. In next step approximately a total of 41 articles relevant to the clinical properties of valproic acid were selected and reviewed correspondingly.
3. Results
Dosage modification should be based on the decision related to prolonged or short-term management [5-7]. All forms of drug such as divalproex sodium, sodium valproate and valproic acid distribute comparable amounts of the dynamic valproate ion. Dissimilarities in the rate of dissolution and following bioavailability of the VPA forms of divalproex sodium necessitate dissimilar overall daily doses of the drug to attain similar steadystate plasma levels of valproic acid. At recommended serum concentrations of 50 - 100 mg/mL, it is a widely used to treat epilepsy and bipolar disorder. Peak plasma concentrations or Cmax are reached in 1 - 4 hours after oral form of the sodium salt or VPA, in 3 - 5 hours for delayed-release, and in 4 - 17 hours subsequent to extended-release tablets. More than a few days might be requested to see the complete property of a given prescribed amount. In complex partial seizures, simple and complex absence seizures, the starting dosage of all of the VPA products is 10 - 15 mg/kg/day with a maximum dosage of 60 mg/kg/day and for migraine prophylaxis is 500 - 1000 mg/day. For chronic prescription related to bipolar disorders a minimum of 250 mg/day to 3000 mg/day with a vigilant monitoring of drug concentration in blood might be the option for a suitable treatment. For acute treatment of bipolar disorders the minimum dose would be 1000 mg a day.
Table 1 shows the kinetic behavior of this drug after prescription. With a half-life of 9 - 16 hours, it appears that the drug follows a variable absorption pattern with a protein binding (PB) concentration-dependent, from 90% at 40 mg/mL to 81.5% at 130 mg/mL. The pattern of metabolism is 30 - 50% and over 40% glucuronide conjugation and mitochondrial β-oxidation respectively. It seems hemodialysis could decrease concentration of drug in whole blood by 20%. Therefore patients with hemodialysis might need higher doses of drug to control seizure attack. Epileptic convulsions could be apparent by a range of clinical norms. Suitable management should be based on individualized epileptic patient that usually take into explanation related to all the three features of analysis of seizure category and epilepsy pattern, reality of comorbidity interaction between associated drugs. For more than 4 decades VPA stipulate in clinical practice as an anticonvulsant to control seizures in forms of tonic-clonic (grand mal), complex partial and juvenile myoclonic. Published report by International League Against Epilepsy (ILAE) authorizes the efficiency and value of ethosuximide and VPA for children with absence seizures as initial monotherapy [2-12]. Karlov et al. [11] reported a prescription based on valproate in dose 15 - 25 mg/kg/day and levetiracetam in dose 20 - 25 mg/kg/day that were used in the treatment of 23 patients with juvenile myoclonic, juvenile absence, and convulsive idiopathic generalized and children absence epilepsy. Optimistic modifications in the incidence of convulsions and epileptic action observed in the electroencephalography (EEG) [11].
| Pharmacokinetic Data [ |
|---|
Valproate is the drug of main option for management in juvenile absence epilepsies (JAE) accompanied by generalized tonic-clonic seizures. If the patient does not appropriately answer, then prescription of lamotrigine or ethosuximide is suggested [13]. However in treatment of cortical myoclonus levetiracetam is first-line treatment but VPA and clonazepam are regularly prescribed. The efficacy of VPA in cortical-subcortical myoclonus has been reported recently by Caviness and Brown [14]. In cortical myoclonus, the most effective drugs are VPA, clonazepam, levetiracetam and piracetam. For corticalsubcortical myoclonus, VPA is the drug of choice. Lamotrigine can be used either alone or in combination with VPA. Levetiracetam or zonisamide can also be used as adjunct therapy with VPA [14, 15]. The risk of seizures differs between 60% and 100% with low grade gliomas and between 40% and 60% in glioblastomas. An innovative and thrilling imminent is the likely involvement of VPA to prolonged survival, mainly in glioblastomas [16]. For the treatment of absence epilepsy, it seems that older AEDs such as ethosuximide and VPA are more efficacious than newer AEDs. Due to reduced side effects, ethosuximide remains the first line treatment for childhood absence epilepsy [2-4].
According to expert opinion published recently, VPA might be the most effective drug in idiopathic generalized epilepsies. It should be avoided in women of childbearing age due to its safety profile. In patients with secondary generalized tonic-clonic seizures, AEDs for which the impact on this seizure type has been formally assessed and which have established superior efficiency than placebo might preferentially be used, such as lacosamide, perampanel and topiramate [17]. In juvenile myoclonic epilepsy, lack of response to VPA was noted in 19% of patients [18]. Corresponding psychiatric disorders demonstrated major association with lack of answer to VPA. The neuroprotective properties of drug have been established in numerous forms of severe central nervous system damages, such as stroke, traumatic brain injury and spinal cord injury. The drug preserves the brain from impairment development via anti-inflammatory, antiapoptotic and neurotrophic effects [19-22]. Elderly are usually more at risk to the adverse effects of VPA than younger adults. Therefore, in this population, the drug should be started at low dosages and titrated gradually. Some of the most troublesome side effects of VPA could be mentioned as: sedation, cognitive side effects and osteoporosis. VPA-drug interactions should be given particular deliberation. It is well known that the pharmacokinetics of all AEDs is altered in the elderly. As renal and metabolic clearance changes in elderly patients, therefore envisaging pharmacokinetic variation seems to be difficult. It seems that there is no agreement on whether VPA should be thought as the ideal first-line management in the elderly [20]. Table 2 shows some of the most clinical properties related to VPA. Due to interand intra- individual variation drug dosing depends on maintenance or acute application in relation to drug concentration monitoring. In combination therapy as a mood stabilizer, lithium might offer a proper synergistic effect. For glutamate excitotoxicity [21], amyotrophic lateral sclerosis [23], Huntington’s disease [24], valproate might provide better clinical efficacy. Following severe intake of a large dose of VPA, central nervous system depression, could be categorized from drowsiness to coma. Respiratory depression, although not a common finding, has also been reported. A vigilant dosing of amitriptyline with VPA co medication is recommended since this addition may guide to a significant raise of amitriptyline serum level [2-4]. In combination with carbapenems, monitoring of VPA levels seems to be necessary [25]. Simultaneous prescription with olanzapine significantly decreases serum concentrations of VPA. Smoking also decreases the levels of VPA [26]. The prescription of this drug in pregnancy could increase the risk of spina bifida, cognitive impairments and many other defects by three times. VPA could increase the risk of autism. A high dose of folic acid is recommended for pregnant women. Second trimester ultrasound scans and alpha fetoprotein measurements seem to be necessary. The drug may have an effect on embryonic heart development and may be associated with congenital cardiovascular defects. The drug is an antagonist of folate and could cause neural tube defects that could lead to lower intelligence quotient scale (Iqs) [27-29].
| Clinical properties of VPA [ |
|---|
The relationship of 25 (OH)D3 levels with anticonvulsant showed that the possibility of vitamin D deficiency could be considered in pediatric patients taking anticonvulsants if they have mental retardation or developmental delay or if they have been taking anticonvulsants for more than 2 years or taking hepatic enzyme inducing drugs [30]. Also there is a significant alteration in trace element status with VPA [31]. A notably small concentration of histamine associates with drug resistance and specifies participation of the H1/H3 receptors [32]. It seems that overweight patients with epilepsy treated with VPA are at superior possibility of metabolic syndrome than individuals who are just overweight. Therefore, the homeostasis model assessment index should be checked in overweight patients who obtain VPA treatment, rather than observing body weight only [33].
4. Discussion
Several forms of epilepsy seem to be the most severe brain complaints that prevailing seizure, autonomic movement, reduced awareness and many other symptoms [33]. Related to the prevalence of epilepsy it seems that it might be affected many people (more than 40 million) of all ages worldwide [34]. The etiology of epilepsy could be an imperative crucial feature in its management and prediction. Analytical and curative progresses recommend that the fundamental division, management and prediction of the population with epilepsy may have undergone some variation. A recent publication by American Association of Poison Control Centers, reported 9,069 human ingestions of VPA in 2004 [2-7].
The wide spectrum antiepileptic drug, sodium valproate (VPA) is generally used for different categories of convulsions such as complex partial seizures, simple and complex absence seizures and monotherapy should be acknowledged as the most therapeutic strategies in epileptic population. An abnormal alteration in gene function could be supposed to be concerned in a broad range of human disorder as well as behavioral, cognitive and neurodegenerative pathologies. Therefore assessment of VPA doses seems to be mostly on the patient’s history of disease and the category of product that could be linked to pharmacokinetic (PK) parameters. According to a recent report for time to twelve month remission, the multivariable figure included first degree relative with epilepsy, age at randomization, neurological insult, number of tonic-clonic seizures before randomization, seizure type and treatment. The figure for time to twenty- four month remission was similar and also included febrile seizure history. The multidrug resistance protein 2 gene may determine individual susceptibility to adverse drug reactions in the central nervous system by limiting brain access of VPA [2-7, 35-37].
Related to the efficacy of VPA for the long-term management of bipolar disorder, there is inadequate reported data. Polytherapy based on simultaneous use of amitriptyline with VPA needs therapeutic drug monitoring because this combination may lead to an extraordinary increase of amitriptyline serum levels [38]. Concurrent use of VPA and carbapenem antibiotics could cause decrease in the VPA levels. The scope of this reduction seems to be greater in patients with meropenem than imipenem or ertapenem [25].
In patients with severe liver damage, pancreatitis, human immunodeficiency virus depression of bone marrow, coaglation hematological disorders should be contraindicated. Liver, pancreas and hematological dysfunction appear to be common. Weight gain and dyspepsia could be mentioned as one of the main important side effects associated to sodium valproate cause. Fatigue, peripheral edema, acne, feeling cold, tremors, blurred vision hair loss dizziness, and headaches could be mentioned as less common side-effects. Sodium valproate might cause an increase in the level of ammonia in the blood lead to hyperammonemia.
In this circumstance the patient might suffer from vomiting and sluggishness, mental change and brain damage. Even when the blood concentration is reported as normal range, this drug could cause hyperammonemia and encephalopathy. In these patients lactolose could not alleviate the hyperammonemia and L-carnitine is used in toxicity related to valproate. Prescription of activated charcoal and gastric lavage could be useful if carried out within one hour of intake. There is also publication that reported brain encephalopathy without hyperammonemia or elevated levels. However polymorphisms in the genes encoding carbamoyl-phosphate synthase 1 and N-acetylglutamate synthase might be risk factors for the development of hyperammonemia during valproic acid- based therapy but carbamoyl-phosphate synthase polymorphism may not be associated with the development of hyperammonemia in Japanese population [2-7, 39-41].
Concurrent use of VPA with olanzapine significantly decreases serum concentrations of olanzapine. In these patients the higher doses of olanzapine should be considered [26]. Tremor, stupor, respiratory depression, coma, metabolic acidosis and death have been reported related to higher trough levels. VPA increases the amount of carbamazepine-epoxide by disturbing its elimination. The cutoff point forecasting hyperammonemia seems to be VPA dose of 30.4 mg/kg/day and VPA C0 of 90.9 mg/mL [1-41].
Finally, in order to highlight the importance of information, for the best individual clinical pharmacotherapy recommendation, VPA treatment strategies in Iranian epileptic population should be accompanied by many factors such as; 1) type of epilepsy, 2) VPA dose and formulation, 3) decision for monotherapy or rational polypharmacy 4) inhibition or induction of CYP450 5) addition of other drugs to VPA 6) an overall prediction of VPA individualized clinical pharmacokinetics.