1. Background
From ancient time, people using plants as parts of food or medicine with varying success to cure and prevent diseases [1]. Traditional medicines are wealthy source of metabolites that are potential source of drugs and essential oils [2].
Medicinal plants have effective and useful compounds which in recent researches focus them for various remedial purposes. Endophytes, are microorganisms which reside in plant tissues and have potential in producing novel metabolites for exploitation in medicine [3]. Each plant species may be host to a number of endophytes [4]. Endophytes have been most extensively studied for their ability to produce antibacterial, antiviral, anticancer, antioxidants, antidiabetic and immunosuppressive compounds [5].
Endophytes have been found in all parts of plant. The scientific community in searching for new therapeutic alternatives has studied and found variable bioactive metabolites in endophytes such as antiviral, anticancer, anti-diabetic and antibacterial compounds. During the long co-evolution of endophytes and their host plants, endophytes have adapted themselves to their special microenvironments by genetic variation, including uptake of some plant DNA into their own genomes [6].
Human often face problems of the tremendous increase in the incidence of fungal or bacterial infections in the world’s population. Chemical synthetic drugs with many side effects are being used to cope with these medical problems. Both human pathogens and phytopathogens are prone to develop drug resistances to decrease substantially the effectiveness of old antibiotics [7]. Antimicrobial resistance is a global quandary demanding urgent action to clearly understand the implications of resistance and to effectively manage patients infected with resistant pathogens, it is important to understand the epidemiology of resistant pathogens, the mechanisms of resistance and treatment options available. Because of the development and spread of drug-resistant pathogens, infectious diseases remain a global problem [8]. Accordingly, there is an urgent need to work towards the invention of safer antibacterial agents.
In Iran, extracts from many types of local plants are used in traditional manner for treatments of various ailments. Little information is available on the occurrence as well as on the potential significance of bacterial endophytes from medicinal plants Zataria multiflora, Achillea willhelmsii and Calendula officinalis L. that seem to be promising medicinal herbs.
2. Objectives
Thus, in this study, we focus on the isolation of bacterial endophytes from these medicinal plants and screening them for activities against some field isolates of human bacterial pathogens such as Staphylococcus aureus, Acinetobacter baumannii, Enterococcus faecalis and Pseudomonas aeruginosa.
3. Methods
3.1. Collection of Plant Samples
In this descriptive study random samples from asymptomatic leaves and branches of three medicinal plants: Zataria multiflora, Achillea willhelmsii and Calendula officinalis L. were collected from Chaharmahal Va Bakhtiari of Iran in Spring 2013.
Leaf and branch portions were thoroughly washed in running tap water, after which they were surface sterilized by submerging them in 70% ethanol for 2 minutes. The portions were further sterilized sequentially in 5.3% sodium hypochlorite solution for 5 min, and 75% ethanol for 0.5 min. After drying, each leaf was divided into segments.
For isolation of endophytic bacteria, the disinfected portions of Achillea willhelmsii and Calendula officinalis L. were distributed onto the isolation media, yeast extract agar (yeast extract 5 gr/L, glucose 10 gr/L, agar 16 gr/L) (YEA) and peptone agar (15 g/L peptone and 15 g/L agar) (PA) while for Zataria multiflora the portions were distributed onto blood agar plates containing 5% ovine blood because of the lack of success on the former medias.
All plates incubated at room temperature for 3 to 7 days [3]. Preliminary bacterial identification was done using Gram staining, catalase activity and biochemical tests on demand.
3.2. Bacterial Strains
Staphylococcus aureus, Acinetobacter baumannii, Enterococcus faecalis and Pseudomonas aeruginosa were collected from hospitals in Isfahan and Shahr-e-Kord. Biochemical examinations including; lysine iron agar (LIA), lysine decarboxylase (LDC), urease, oxidation-fermentation (OF) and triple sugar iron agar (TSI) tests beside gram staining, growth on MacConkey agar and cetrimide media, also oxidase and catalase examinations were followed for confirmation of the isolates. The methods for isolation and identification of all isolates were based on Quinn et al. guidelines [9].
3.2.1. Endophytic Bacterial Contents
For examination of antibacterial activities of endophytic bacterial contents, selected colonies of isolated endophytic bacteria were diluted in peptone water (0.1%) and displayed as drops (Pasteur pipette) in PA and YEA media. Petri dishes were incubated at room temperature at 37°C for 24 - 48 hours. The bioassays were conducted with using growing colonies in PA and YEA media, then inactivated them by chloroform (15 minutes.).
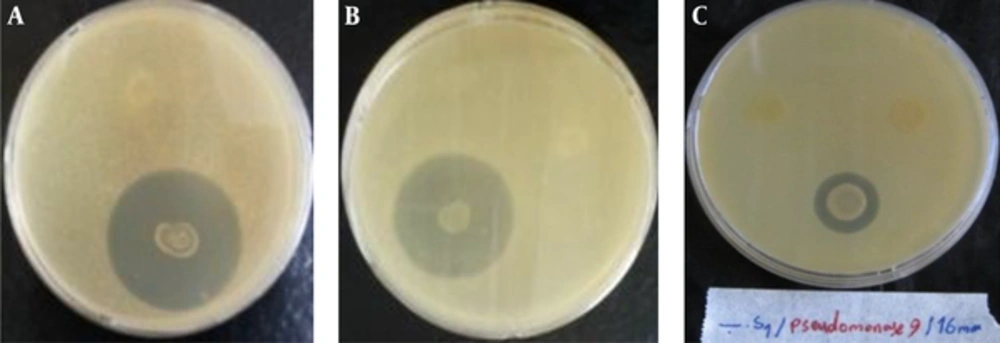
Plates were opened (30 minutes) to evaporate the substance. At the same time, the reactivation of forty field isolates of Staphylococcus aureus, Pseudomonas aeruginosa, Acinetobacter baumannii and Enterococcus faecalis (each 10 isolates), (BHI agar 24 hours/37°C) were made. 200 μL of the each culture properly reactivated were transferred to 10 mL of semi-solid BHI medium and shaken. This mixture was deposited onto the surface of plates (YEA) containing chloroform inactivated bacterial colonies. The plates were incubated (37°C/24 hours) for the observation of inhibition halos [3] (Figure 1).
3.2.2. Endophytic Bacterial Broth Culture
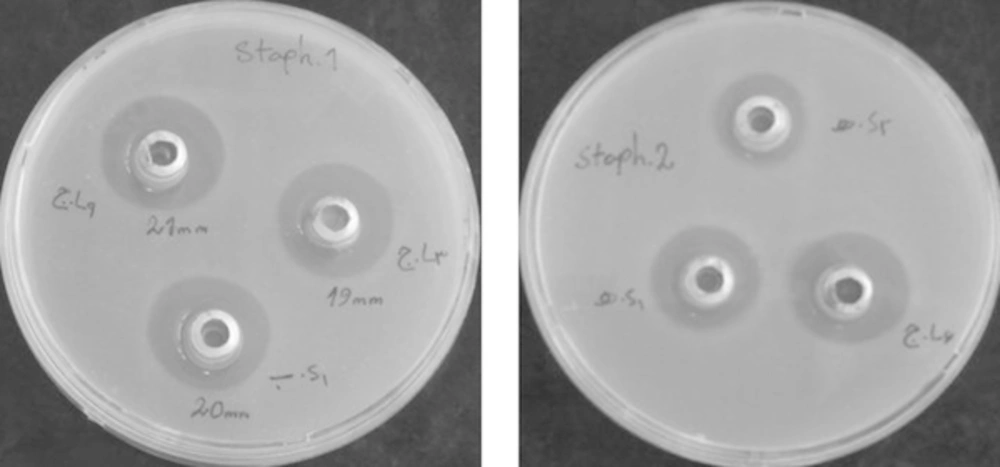
To test antibacterial activity of endophytic bacterial culture broth, briefly, 200 μL of each field isolate (108 cfu/mL) was added into 15 mL of YEA at 50°C, mixed thoroughly and poured into a 9-cm diameter of petri-dish. After solidification, two to three sterilized stainless cylinders (5-mm internal diameter and 10-mm high) were placed open end up on each plate. The culture broth of endophytic bacterial isolates grown in LB broth (18 - 24 hours incubation at 37°C), centrifuged at 10000 rpm for 15 minutes and filter-sterilized supernatants (100 μL of each) were poured in cylinders on each bacterial plate [10] (Figure 2).
4. Results
Segments of surface sterilized leaves, and stems of Zataria multiflora, Achillea willhelmsii and Calendula officinalis L. incubated on yeast extract agar, peptone agar and blood agar plates showed growth of morphologically distinguishable bacterial colonies surrounding the segments after 24 - 48 hours. A total of 9 phenotypically distinguishable bacterial endophytes were isolated in pure from 3 medicinal plants. Regarding the herbs out of these 9 isolates 2 were from Zataria multiflora (only stem), 2 from Achillea willhelmsii (1 branch, 1 leaf), and 5 from Calendula officinalis L. (3 leaf, 2 branches).
The bacterial endophytes were characterized based on micromorphological, gram staining and catalase examinations. Out of 9 bacterial endophytes, 4 were Gram-positive (2 cocci and 2 Bacilli) and 5 were Gram-negative (1 Bacilli, 2 cocci and 2 Coccobacilli). Filamentous forms were not detected in any of the plant samples.
Antimicrobial activities of all bacterial endophytes were assessed against forty bacterial field isolates of Staphylococcus aureus, Pseudomonas aeruginosa, Acinetobacter baumannii and Enterococcus faecalis (each 10 isolates). The isolate which inhibited growth of any of the test isolate(s) was considered having antibacterial activity and the length of inhibition zone was measured (Tables 1, 2).
| Herb | Average Inhibition Zone (mm), Mean ± SD | |||||
|---|---|---|---|---|---|---|
| Endo. | Morph. | S. aureus | A. bumani | E. faecalis | P. aeruginosa | |
| Z.mutiflora | 1B | G- cocco bacilli | 5.2 ± 1.65 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 2B | G- cocco bacilli | 8.4 ± 1.82 | 0 ± 0 | 0 ± 0 | 2 ± 1.26 | |
| A. willhelmsii | L8 | G- cocci | 15.4 ± 0.77 | 0 ± 0 | 7 ± 2.22 | 6.7 ± 2.15 |
| 1B | G+ Bacilli | 15.2 ± 1.04 | 0 ± 0 | 5.2 ± 2.03 | 7.1 ± 2.96 | |
| C.officinalis L. | 1B | G+ coccci | 15.5 ± 0.53 | 0 ± 0 | 6.5 ± 2.12 | 7.3 ± 3.07 |
| 2B | G+ coccci | 15.2 ± 0.9 | 0 ± 0 | 7.6 ± 1.97 | 7.6 ± 2.78 | |
| 2L | Bacilli | 2.8 ± 1.77 | 0 ± 0 | 0 ± 0 | 5.5 ± 2.28 | |
| 3L | G- cocci | 15.6 ± 0.62 | 0 ± 0 | 7.7 ± 2.02 | 8.5 ± 3.30 | |
| 4L | G+ Bacilli | 14.5 ± 0.93 | 0 ± 0 | 6.5 ± 2.07 | 5.2 ± 2.18 | |
Abbreviations: Endo, endophytes; morph, morphology; L, leaf; B, branch.
Out of 9 endophytes screened, chloroform inactivated colonies of 1 endophytes from branches of Achillea willhelmsii and one from branches of Zataria multiflora showed average inhibition zone of more than 15 mm against Staphylococcus aureus isolates (Table 2), while supernatant culture broth of 1 endophyte from leaves and 2 endophyte from branches of Calendula officinalis L. 1 endophyte from leaves and 1 endophyte from branches of Achillea willhelmsii showed average inhibition zone of more than 15 mm against Staphylococcus aureus isolates (Table 1).
| Average Inhibition Zone (mm), Mean ± SD | ||||||
|---|---|---|---|---|---|---|
| Herb | Endo. | Morph. | S. aureus | A. bumani | E. faecalis | P. aeruginosa |
| Z.mutiflora | 1B | G- cocco bacilli | 0 ± 0 | 0±0 | 0±0 | 0±0 |
| 2B | G- cocco bacilli | 22.3 ± 3.89 | 2.8 ± 1.36 | 9.1 ± 4.31 | 9.1 ± 3.60 | |
| A. willhelmsii | L8 | G- cocci | 2 ± 1.26 | 2.3 ± 0.89 | 1.8 ± 1.17 | 0 ± 0 |
| 1B | G+ Bacilli | 20 ± 1.33 | 9.3 ± 2 | 11.1 ± 1.84 | 9.6 ± 1.90 | |
| C.officinalis L. | 1B | G+ coccci | 0 ± 0 | 1 ± 0.94 | 0 ± 0 | 2.2 ± 2.08 |
| 2B | G+ coccci | 0 ± 0 | 0±0 | 1 ± 0.94 | 0 ± 0 | |
| 2L | Bacilli | 0.8 ± 0.75 | 1.7 ± 1.09 | 0 ± 0 | 0 ± 0 | |
| 3L | G_ cocci | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| 4L | G+ Bacilli | 0 ± 0 | 2.9 ± 1.55 | 1.1 ± 1.04 | 0 ± 0 | |
Abbreviations: Endo, endophytes; morph, morphology; L, leaf; B, branch.
Chloroform inactivated colonies of 1 endophytes from branches of Achillea willhelmsii and 1 from branches of Zataria multiflora showed average inhibition zone of more than 8 mm against Enterococcus faecalis isolates (Table 2), also 1 endophytes from branches of Achillea willhelmsii showed average inhibition zone of more than 8 mm against Acinetobacter baumannii.
Chloroform inactivated colonies of 1 endophytes from branches of Achillea willhelmsii and 1 from branches of Zataria multiflora showed average inhibition zone of more than 8 mm against Pseudomonas aeruginosa isolates (Table 2), while supernatant culture broth of 1 endophyte from leaves of Calendula officinalis L. showed average inhibition zone of more than 8 mm against Pseudomonas aeruginosa. Also 1 endophyte from branches of Zataria multiflora and 1 endophyte from leaves of Calendula officinalis L. showed average inhibition zone of more than 8 mm against Staphylococcus aureus.
5. Discussion
In the present study, we focus on the bacterial endophytes which were isolated from stems and leaves of Zataria multiflora, Achillea willhelmsii and Calendula officinalis L. although endophytes could also exist in root, flower, seed and fruit.
A total of 9 endophytes were isolated from three plants found in Chaharmahal Va Bakhtiari province. Calendula officinalis L was found to host the highest number of endophytes (5 isolates).
Generally, 1 endophyte isolated from stem of Achillea willhelmsii and 1 endophyte isolated from stem of Zataria multiflora found to be more effective against Staphylococcus aureus than other endophytes (Table 2). These plants are used for treatment of various ailments by local people, so we suggest more particular studies on endophytes of these medicinal plants.
In according to these results, other studies showed that endophytes are a good source of antibacterial agents [11]. There are some studies on isolating and detecting antimicrobial activities of fungal and bacterial endophytes from other medicinal plants. In our earlier work [12] we isolated 8 endophytic fungi and 7 endophytic bacteria from 5 medicinal plants and evaluated their activities.
Sette et al. [13] isolated 25 fungal endophytes from Coffea Arabica and 14 fungal endophytes from Coffea robuta, then studied molecular characterization and antimicrobial activities of the isolated endophytes.
Hazalin et al. [14] isolated and identified 300 endophytes from 43 plants and studied cytotoxic and antibacterial activities of them.
In a study by Ding et al. [15] an endophytic bacteria were isolated from the mangrove tree Kandelia candel. The metabolites isolated from culture broth of this endophyte showed broad antimicrobial effect against methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus faecalis. In our study, both secreted metabolites and also structural constituents of isolated endophytic bacteria showed beneficial effects against Staphylococcus aureus but relatively weak inhibitory effect against Enterococcus faecalis was found.
In a study by El-Shatoury et al. [16] a total of 25 endophytes were isolated from Achillea fragrantissima that one in Streptomyces genus, showed a broad antimicrobial activities against pathogenic bacteria Staphylococcus aureus, Salmonella typhimorium and E-Coli. Since Achillea fragrantissima and Achillea wilhelmsii belong to one genus their results can be considered to be in line with our results for Achillea willhelmsii against indicator bacteria.
Sunkar and Nachiyar [17] by studying on the Brassica oleracea, showed that endophytic bacteria of this plant have antibacterial activity against Staphylococcus aureus and Salmonella typhi. Regarding Staphylococcus aureus their results correspond with the results of this study.
Interesting finding in the present work is isolation of one endophytic Gram positive bacilli from branches of Achillea willhelmsii that in both examinations (supernatant broth and inactivated colony) showed considerable effects against nearly all examined bacterial isolates (Tables 1, 2). We suggest more detailed studies in this regard.
The results of the present work suggest examined plants are good source for searching endophytic microorganisms having the potential of natural compounds that can be used in agriculture, medicine and pharmaceutical industry.