1. Background
The species in the genus Mycobacterium are acid fast and they inhabit in various environmental reservoirs such as, ground, tap water, soil, animals, and humans. They are grouped among the actinomycetes and include non-pathogens as well as highly successful pathogens e.g. Mycobacterium tuberculosis, Mycobacterium leprae and Mycobacterium ulcerans, the etiological agents of tuberculosis (TB; 10 million new cases per year develop active disease), leprosy and Buruli ulcer (the third most common mycobacterium infection), respectively [1-3].
Mycobacterium marinum is a close genetic relative of M. tuberculosis as they share more than 85% amino acid identity (a similarity of 99.3 % in 16S rRNA sequence) and very closely related, both in its pathology and genetically, to M. ulcerans about more than share 99.6 % amino acid identity. M. marinum is an attractive model system for identification, study of virulence factors, disease development, and drug resistance in M. tuberculosis. Moreover, M. marinum causes a systemic TB-like infection in ectodermic hosts e.g. fish and frogs. The infections caused by M. marinum in warm-blooded hosts, such as human, and poikilothermic animals, such as fishes and frogs, is indistinguishable with the infection caused by M .tuberculosis complex [4, 5]. In comparison to M. tuberculosis, M. marinum grows with a relatively short doubling time and growth is limited at higher temperatures, making M. marinum easier to handle than e.g. M. tuberculosis and study in the laboratory [6].
Microbial population encounter different environmental stresses and usually adaptation to these stresses causes extended tolerance to multiple other lethal stressors. This phenomenon is defined as stress hardening, which refers to the increased resistance to lethal factors after adaptation. Like other bacteria, Mycobacterium spp. competes for nutrients, and they have developed sophisticated ways to adapt to different environmental stresses. Phosphorylation dependent signal systems and alternative σ-factors play essential roles as switches to alter gene expression patterns globally [7]. Although there are some progress in understanding the stress responses in Mycobacterium spp. even at molecular level, but there are little information concerning the effects of environmental stresses on growth, cell division and biochemical characteristics of M. marinum.
2. Objectives
The aim of this study was evaluating the effects of some environmental stresses such as pH, oxidative, temperatures and osmotic pressure on growth, biofilm formation, and biochemical characteristics of M. marinum CCUG 20998.
3. Methods
In this descriptive-analytic study M. marinum CCUG 20998 was subjected to different conditions of environmental stresses such as pH, oxidative, osmotic pressure, and temperatures. The growth data were analyzed by measuring colony forming unit (CFU) using SPSS software version 19. All media and materials used in this study were obtained from (Merck Co. Darmstadt, Germany). Middlebrook 7H10 and 7H9 and Middlebrook OADC Enrichment were obtained from BD BBLTM Co. New Jersey USA.
3.1. Bacterial Strain and Culture
Mycobacterium marinum CCUG 20998 was obtained from culture collection, university of Goteborg, (CCUG, Goteborg, Sweden) used through this study. Lyophilized vial was cultured in Middlebrook 7H10 medium containing Middlebrook OADC enrichment. Incubation was performed at 30°C for 7 - 10 days until visible colony formation. For determining the mid-log phase, a single colony grown on Middlebrook 7H10 was inoculated in 7H9 Middlebrook medium. Optical density of growth was measured at 600 nm every 24 hours until the cells reach to mid-log phase. The cells at mid-log phase were harvested by centrifugation at 3,000 g at 4°C for 90 minutes. The harvested cells were washed three times in 5 mL of phosphate buffer (PB) and resuspend in 10 mL of pre-chilled (4°C) 0.1 M phosphate buffer (PB) and kept in refrigerator for evaluating the effects of different stress conditions.
3.2. Condition Used for Different Stresses
For evaluating different stresses, M. marinum CCUG 20998 cells in pre-chilled phosphate buffer was grown in 7H9 Middle brook broth medium to an optical density at 600nm of 0.2 - 0.4. For treating acidic or basic stresses, 50 micro liter of cell suspension inoculated in 7H9 Middlebrook medium having pH (3.0, 5.0, 7. 0, 9 .0, 1.0 and 11. 0), pH values were adjusted using 0.5 M HCl and 1 M KOH, respectively. Incubation was continued for additional 24 hours before harvesting the cells. In order to assess osmotic pressure, M. marinum CCUG20998 cells in mid-log phase were transferred in to distilled water containing sodium chloride at concentrations of 0.0 %, 2%, 4%, 6%, 8% (w/v) for two hours. For oxidative stress M. marinum CCUG20998 cells in mid-log phase were treated with H2O2 at concentrations of 600, 1200, 2400, 4800 and 9600 (ppm), for two hours. For heat stress the cultures were shifted to 32.5, 42.5, 52.5, 62.5, 72.5, 82.5 and 100°C, respectively, and incubated for an additional 24 hours before harvesting, as described above (centrifugation was performed at 4°C).The stress-free control was prepared by inoculating M. marinum CCUG 20998cells in pre-chilled phosphate buffer by transferring into pre-warmed 7H9 Middlebrook medium.
For evaluating the effect of each stresses on growth patterns, stressed cells were centrifuged at3000 rpm for 2 hours at 4°C. Cell pellets were resuspended in 50 micro liter of 7H9 medium and inoculated in 100 mL- Erlenmeyer containing 25 mL fresh 7H9 Middlebrook medium. Incubation was carried out for 120 hours.
The pattern of growth was measured (OD 600nm) with 24 hours intervals. Biochemical characteristics such as, Tween hydrolysis, nitrate reduction, aryl sulfatse, urease, telluride reduction, salt tolerance, semi quantitative catalase, niacin production, acid phosphatase, pyrazinamidase and other tests were evaluated and compared to non-treated cells.
3.3. Estimation of Biofilm Formation by Stressed M. marinum CCUG 20998
Biofilm formation was determined using Carter et al. method with some modifications [8, 9]. 100 μLof frozen stock culture was inoculated in 10 mL of fresh 7H9 medium with OADC and Tween 80 and incubated at 30°C with agitation for seven days. 200 µ of the cell suspension were added to the wells of a 96- well flat bottom polystyrene micro titer plate in triplicate (MicroWell TM Plates Nunclon TM Nunc Nuncleon, Rosklide, Denmark), and incubated at 30°C without agitation in a sealed container with 20 mL sterile distilled water to prevent drying. Media without bacteria were used as negative controls on each plate. After incubation for two weeks, bacterial growth was determined by OD 600 measurement. The wells were washed once with 250 µL tap water, and the remaining biofilm was stained using 250 µL l 1% crystal violet, followed by 30 minutes incubation at room temperature. The wells were rinsed three or four times with tap water. The stained biofilm was resuspended in 250 µL ethanol: acetone 70:30. Finally, the amount of biofilm was measured at OD600. Results were presented as the medium value of triplicate, subtracting the medium value for the negative control.
4. Results
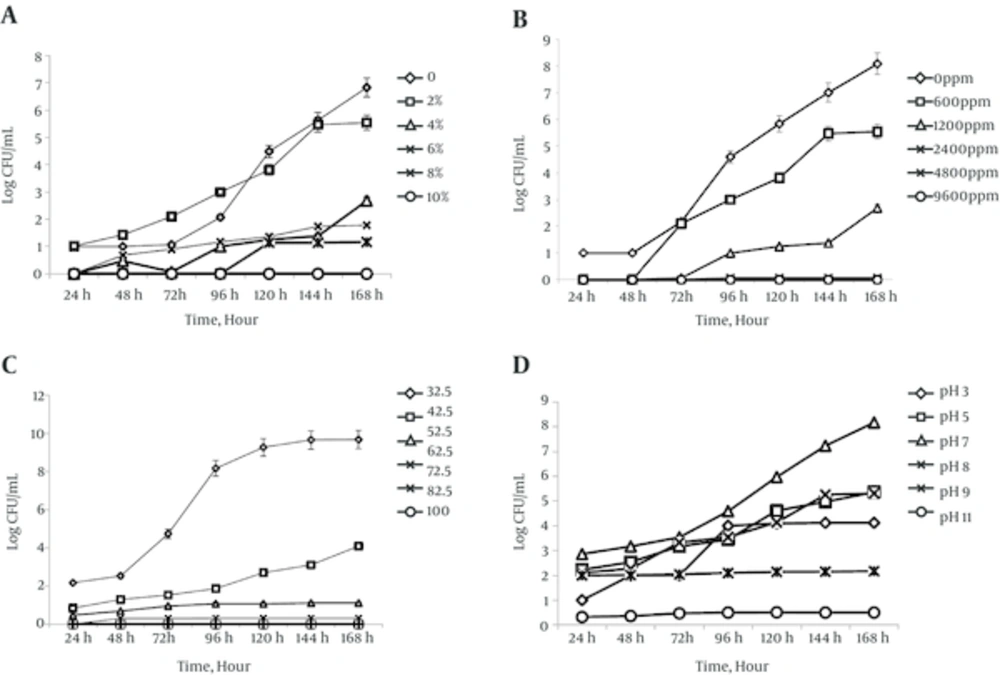
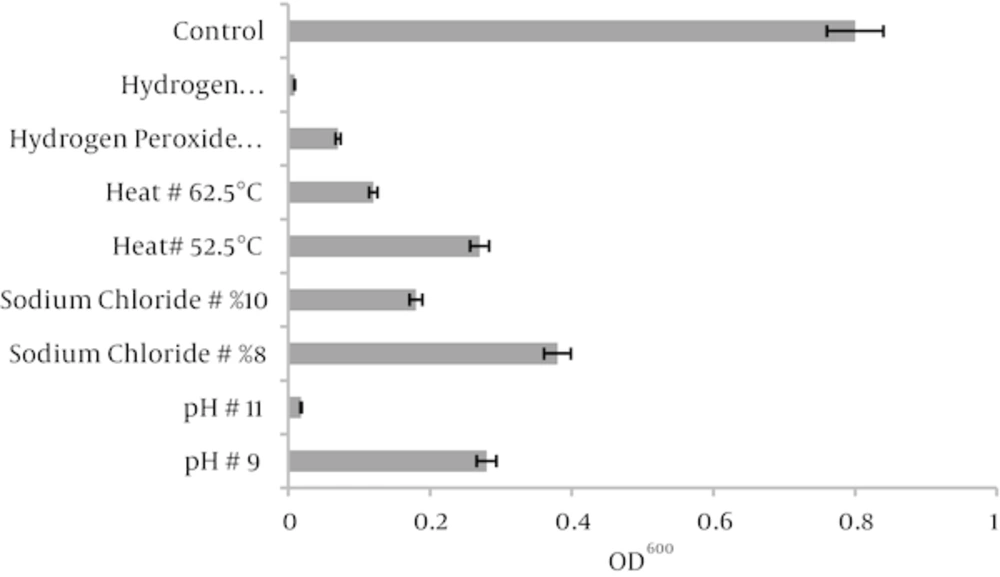
In this study exponential phase of Mycobacterium marinum CCUG 20998 cells were subjected to a variety stress conditions such as different sodium chloride, hydrogen peroxide concentrations, and different pH and temperature values. Figure 1A shows growth pattern of M. marinum CCUG 20998 cells after treating with concentrations of 0.0 %, 2%, 4%, 6%, 8% (w/v) sodium chloride in distilled water. The effect of different concentration of hydrogen peroxide, different temperatures, and pH on growth pattern of M. marinum CCUG 20998 is shown in Figure 1B, C and D, respectively. As shown in Figure 1A sodium chloride at %10 concentrations inhibited the growth of M. marinum CCUG 20998 cells. Hydrogen peroxide at 9600 ppm concentration and temperature at 62.5°C could inhibit M. marinum CCUG 20998 growth completely (Figure 1B and 1C), respectively. Maximum tolerance for pH was pH=11 as shown in Figure 1D. The obtained results showed increased tolerance of M. marinum after treating to stress conditions in comparison to non-stressed cells. Table 1 shows the effects of different stress condition on biochemical characteristics of M. marinum CCUG 20998. As shown in the table the most effective stress conditions were pH = 11, sodium chloride (%8 W/V), H2O2 at 4800 ppm and temperature at 52.5°C.M. marinum CCUG 20998 growths was significant at 25 - 32°C temperature ranges. Pigmentation was lost at all stresses condition and cells showed sensitivity to isoniazid upon all stress conditions. Also heat stability, Tween hydrolysis, urea hydrolysis and acid phosphatase activity was negative in M. marinum CCUG 20998 cells treated with different stresses. Biofilm formation of M. marinum before and after stress conditions is shown in Figure 2. As shown there is good biofilm formation by M. marinum CCUG 20998 using 7H9 medium with Tween 80 at 32°C. Biofilm formation by M. marinum CCUG 20998 reduced about 90% in conditions such as pH = 11 and hydrogen peroxide at 9600 ppm concentration.
| Stress Condition | |||||
|---|---|---|---|---|---|
| Characteristics | 1 | 2 | 3 | 4 | 5 |
| Growth at | |||||
| 25 - 27°C | + | + | + | + | + |
| 32°C | + | + | + | + | + |
| 42.5°C | - | - | - | - | - |
| 52.5°C | - | - | - | - | - |
| Pigmentation | + | - | - | - | - |
| Resistence to | |||||
| Isoniazid, 1 µg mL-1 | M | ¬- | - | - | - |
| Isoniazid, 10 µg mL-1 | M | - | - | - | - |
| TCH | + | - | - | - | - |
| Hydroxyamine | + | + | + | + | + |
| p-Nitrobenzoic acid | M | M | M | M | M |
| NaCl | - | - | - | - | - |
| Thiacetazone | M | M | M | M | M |
| Oleate | F | F | - | F | F |
| Catalase activity | |||||
| > 45 mm | F | - | - | - | - |
| Heat stable | + | - | - | - | - |
| Tween 80 hydrolysis | + | - | - | - | - |
| Urease | + | - | - | - | - |
| Niacin production | - | - | - | - | - |
| Nitrate reduction | - | - | - | - | - |
| Acid phosphatase | + | - | - | - | - |
| Arylsulphatase, 3 day | F | - | - | - | - |
| Pyrazinamidase, 7 days | M | - | - | - | - |
a1, Not stressed control M. marinum CCUG 20998 cells; 2, Stressed M. marinum CCUG 20998 cells at (pH = 11); 3, Stressed M. marinum CCUG 20998 cells at 8% NaCl concentration; 4, Stressed M. marinum CCUG 20998 cells at 4800 ppm H2O2; 5, Stressed M. marinum CCUG 20998 cells at 52.5°C.
5. Discussion
In this study, the effects of some environmental stresses on growth pattern, biofilm formation and biochemical characteristics by M. marinum CCUG20998 were investigated. Osmotic pressure, oxidative stress, different pH and temperatures were used. M. marinum CCUG 20998 can tolerate up to 8% W/V sodium chloride concentration in 7H9 medium. Maximum tolerance up to 4800 ppm H2O2was detected in M. marinum CCUG 20998. Also, upon increasing oxidative and pH values more clumping formation detected.
Usually, for survival in the face of stress conditions bacteria may move by swimming using molecular motor and their flagella or may adapt to changes in their immediate vicinity by responding to the imposed stresses. This response is accomplished by changes in the patterns of gene expression for those genes whose products are required to combat the deleterious nature of the stress [10]. Osmolarity is an important criterion to distinguish between the external and internal associated environment around the bacteria [11]. Osmotic fluctuation alter torgur pressure, which can impair protein folding and metabolic activity [12]. Bacteria typically counteract such fluctuations through the compensatory accumulation or expulsion of compatible solutes that restore osmotic balance in the cells. In addition, pathogenic bacteria have virulence-associated osmosensory mechanisms that are triggered at the transcriptional level [13, 14]. It can be conclude that increasing sodium chloride concentration could decrease viability of cells and cell division patterns. Although the members in Mycobacterium genus are acid fast bacteria having a rigid complex cell wall, but it seems they cannot face to increasing osmotic pressure by synthesis of compatible solutes. Recently, Hatzios et al. 2013. reported that osmotic stress stimulates a signaling network in Mycobacterium tuberculosis regulated by the eukaryotic-like receptor PknD (Ser /Thr protein kinase) [14]. Expression of the PknD substrate Rv0516c was highly induced by osmotic stress. Furthermore, Rv0516c disruption modified peptidoglycan thickness, enhanced antibiotic resistance, and activated genes in the regulon of the alternative σ-factor SigF (sigma factor F) .These findings identify an osmosensory pathway or chest rated by PknD, Rv0516c, and Sig F that enables adaptation to osmotic stress through cell wall remodeling and virulence factor production. The widespread occurrence of eukaryotic-like Ser/Thr protein kinases in bacteria, these proteins may play an important role in bacterial osmosensing.
H2O2 is a stable oxidant that reacts with most organic substrates. Many oxidizes in bacterial cells generate superoxide anion, which forms hydrogen peroxide by spontaneous or catalyzed dismutation. It can cross cell membranes easily and the cytotoxicity is due to production of highly reactive hydroxyl radicals that are generated by Fenton’s reaction. Because of the very short half-life of OH by product produced by H2O2 its reactions are diffusion limited, i.e., they take place practically at the site of generation. The cytotoxic nature of superoxide and peroxide, which are mild oxidizing agents, is due to their ability to generate intracellular OH. DNA which is damaged by such free radicals contains a multiplicity of base and sugar derivatizations and most of these are mutagenic. Attack at a sugar leads to sugar fragmentation as well as loss of base resulting in strand break. Radicals can also damage the bases of the DNA such as ring-saturated thymines, hydroxyl methyl uracil, thymine fragments and adenine ring opened products [15, 16]. The results in this study showed that upon increasing oxidative and pH values more clump formation was detected. So, it can be concluded that clump formation is one of the ways that the species in the genus Mycobacterium could response in harsh environments such as exposing to different stress isolated M. marinum from biofilm in water distribution systems, also quantified the biofilm formation by M. marinum and the effect of antimicrobial agents were evaluated on biofilm formation by this organism [17, 18].
Bacterial responses to different stresses conditions have been studied mostly under in vitro conditions. Although the results obtained from these studies have provided useful information on survival under stress conditions and expression of different genes, they might not truly reflect the in vivo situation where host factors also contribute to establishment of the organism during infection. For a better understanding of interaction between host and parasite it is desirable to delineate the bacterial functions that are specifically expressed under in vivo conditions and to assign their role in pathogenesis. Several approaches are now being adopted to identify in vivo expressed genes and examine their functions. The results from such analysis will furnish a clear picture of how animal or human pathogens adapt themselves to in vivo stress situations and provide a better insight into the molecular basis of pathogenicity.