1. Background
Infertility is typically determined as the inability to achieve pregnancy after one year or more of regular and UN protected coitus, so that about 8% - 12% of couples encounter with difficulty in pregnancy around the world [1]. In spite of some problems, there are many benefits in this treatment process, more over approximately 15% - 20% of collected follicles are immature, in the other words, more collected follicles are in germinal vesicle (GV) stage [2, 3]. In vitro techniques were used for research, genetic improvement or commercial purposes and the successful embryo production largely depends on the components of culture media during oocyte maturation [3, 4].
The formation of reactive oxygen species (ROS) is a natural process that happens in the cell during electron transfer reactions. Cellular molecules such as lipids, proteins and nucleic acids are altered by ROS [5]. In the recent studies different antioxidants such as ß-mercaptoethanol (ß-ME), cysteine, cysteamine [6] and anthocyanin were used to supplement IVM of oocyte [7]. In the present time, there is an excessive interest in natural antioxidants instead of synthetic types, which may contribute to prevent oxidative damages [5].
For many years, the medicinal herbs have been used for treatment of several diseases in animals and humans [8]. During the last decades, an increasing interest in natural antioxidants consumption has arisen as a result of emerging concerns related to the synthetic chemicals side effects. This subject has increased the consumption of natural antioxidants derived from plants and marine sources [9, 10]. Recently, seaweeds and marine invertebrates are well recognized as sources of natural metabolite which often suggested against more diseases such as cancer [11].
Scare information is reported about antioxidant properties of crude extracts from marine organisms [12]. Among marine invertebrates, sea cucumbers are well known to demonstrate advantageous effects on human health. Black sea cucumbers or Holothuria leucospilota are marine invertebrates of the phylum of echinoderms with a long body and leathery skin that have been used in Asian traditional medicine since ancient times [13]. Several studies have shown multiple biological activities of sea cucumber such as wound healing and antimicrobial, anticancer, anti-nociceptive and immune-modulatory properties [14].
Holothuria leucospilota generally distributed from the Red Sea to Hawaii, eastern Pacific (Is. Clarion, Socorro, Galapagos); widespread in the tropical Indo-Pacific and Conand ; tropical, Indo-Malayan Region, Pacific Ocean, E Africa, depth range 0 - 10 m.
Recent studies have shown that the use of natural antioxidants could exert significant effect as therapeutic strategy in several human diseases mediated by oxidative stress [15].
It is indicated that during in vitro oocyte maturation, the levels of antioxidants have been reduced as compared with in vivo condition, therefore, the addition of a natural antioxidant to the medium may be appreciable for in vitro oocyte maturation [16].
2. Objectives
Hence, by considering the antioxidant capacity of sea cucumber and lack of documents regarding promoting effect of sea cucumber on oocyte maturation, this study was conducted to determine the effect of sea cucumber alcoholic extract on in vitro maturation of mouse oocyte.
3. Methods
3.1. Animals
In this experimental study, immature female Novel Medical Research Institute (NMRI) mice, 24 - 26 days purchased from Razi Institute, Mashhad, Iran and used in this study. They were housed in 23 - 25°C temperature in a pathogen free condition and in a light-controlled room (12 hours light: dark cycle) and fed pellet food (Javaneh Khorasan, Iran) and water ad libitum. All procedures in this study on animals exerted on the basis of ethic approval from Kharazmi Institute, Islamic Azad University of Mashhad.
3.2. Preparation of Sea Cucumber Extract
Persian Gulf sea cucumber Holothuria leucospilota Brandt (H. leucospilota) was collected from the coast of Qeshm, Iran and immediately put on ice and was transferred to Mashhad university research laboratory. Collected sea cucumbers was dried in darkness after expelling the body contents and then crushed into small segments. For extraction, 10 mL methanol was added to each gram of dried sea cucumber for 72 hours. Then, filtration was performed and extract was concentrated in the vacuum evaporator (Heidolph, Germany) [17].
3.3. Collection of Preantral Follicles
Female mice (24 - 26 days) were euthanized by cervical dislocation and early preantral follicles with diameter, 120 - 150 μm, were mechanically isolated from the ovaries using a 30-gauge needle under a stereomicroscope.
3.4. In Vitro Preantral Follicle Culture
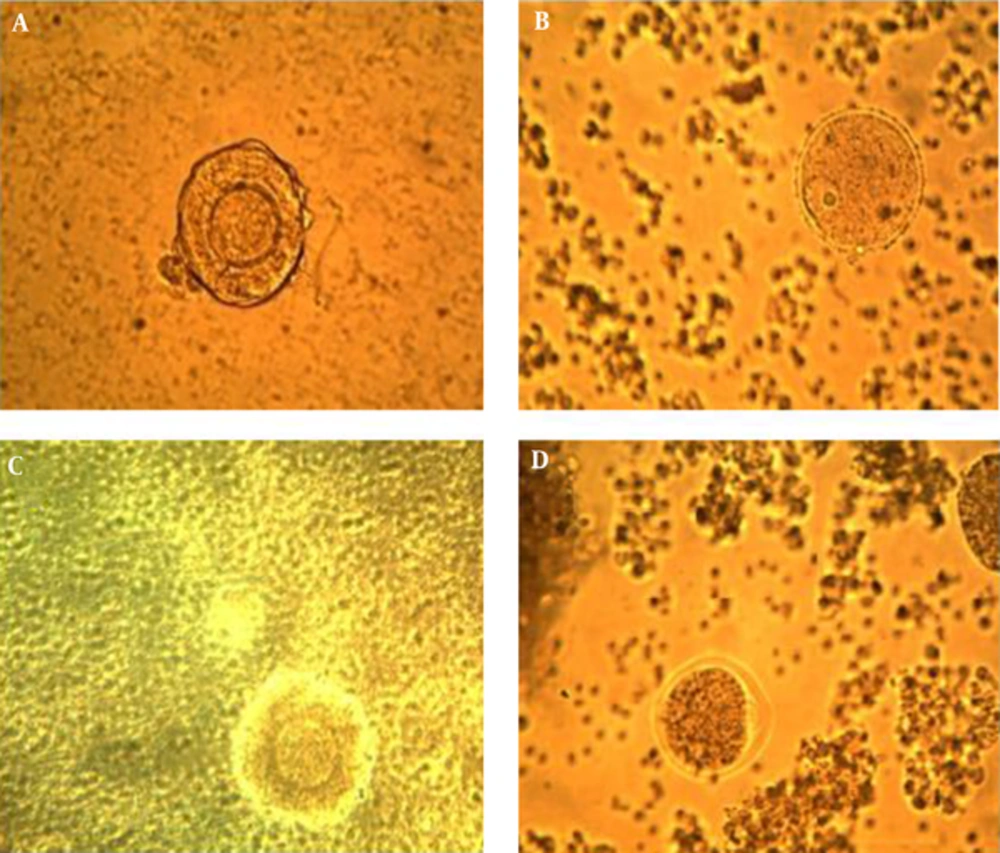
The follicle culture medium consisted of α- MEM Gibco) supplemented with 5% FBS, 100 units/mL penicillin, 100 μg/mL streptomycin, 5 μg/mL insulin, 5 μg/mL transferrin and 5 ng/mL selenium (ITS: Gibco). Then, 100 IU/mL follicular stimulating hormone (rhFSH: MerekSerono-Switzerland) was added to the basal medium in the in vitro follicle culture for developing follicles. Follicles were individually cultured in a 24-well plate containing 1 mL culture medium for 13 days. Subsequently, follicular growth was monitored every 2 days (2, 4, 6, 8, 10, and 12) under an inverted microscope at 200 × magnification. Photomicrograph was captured by a camera system (DP-50: Olympus, Tokyo, Japan) and follicle diameter was measured using Image J software version 1.43 (National Institutes of Health, Bethesda, MD). When follicles clearly formed an antral like cavity on day 12, ovulation and meiotic resumption were induced by refreshing media with culture media supplemented with 5 IU/mL human chorionic gonadotropin (HCG). On day 13, follicles were checked for ovulation 16 h after hCG administration and ovulation was considered when the follicle was visually ruptured and the cumulus oocyte complex (COC) extruded from the follicles [18].
3.5. Assessment of Follicle Parameters
The follicular parameters such as survival, degeneration, % of germinal vesicle and oocyte maturation were determined under inverted microscope during the culture program. At the beginning, granulosa cell proliferation is limited and theca cells grow out to the culture plate. The follicles remain in this stage until day 4 of culture. Marked granulosa cell proliferation and a large preantral follicle define the diffuse stage. From day 4 to day 6 of culture, granulosa cells overgrowth the theca cell monolayer through the basement membrane and the follicles regularly expanded. From day 6 or 8 up to day 12 of culture, the follicles form an antral-like cavity, which characterizes the antral stage. In this culture system, surviving follicles were determined as those that could preserve their oocyte completely embedded within the granulosa cell mass and that exhibited no signs of degeneration [19, 20].
3.6. Determination of Antioxidant Capacity by DPPH Scavenging Assay
Radical scavenging abilities of the extracts were measured by using the stable radical 2, 2-diphenyl-2-picrylhydrazyl (DPPH), based on the method of described previously [21]. The free radical scavenging activity of the extract was determined by using the DPPH assay. Then the methanol extracts (500 μL) was added to 500 μL solution of DPPH in methanol. After the reaction was allowed to take place in the darkroom for 30 min at room temperature, the absorbance at 517 nm was recorded to determine the concentration of remaining DPPH. BHT was considered as positive control. The radical scavenging activity was calculated as follows:
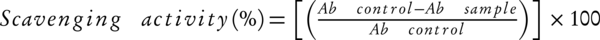
3.7. Evaluating the Expression of TNF-α by Flow Cytometry
For evaluating TNF-α expression in granulosa cells, flow cytometry analysis was used. In this assay, the treated and untreated granulosa cells were trypsinized and transferred to centrifuge in 2000 rpm at 4°C. Then rinsed with PBS and centrifuged again, and were added primary TNF-α antibody (Abcam, UK) (1:100) overnight. On the next day, washing and centrifugation was performed, then secondary antibody conjugated with FITC (1:20) was added for 45 minutes. Finally, 300 µL 0.1 % formalin was added and flow cytometry was conducted using FACS caliber flow cytometry (Becton Dickinson, USA) [22].
3.8. Statistical Analysis
SPSS 16 were applied for all statistical analysis. Data were analyzed by one-way analysis of variance (ANOVA).Significant differences between treatments were determined by Duncan’s multiple range tests. All of the data are expressed based on mean ± SD. P < 0.05 was considered statistically significant.
4. Results
4.1. Assessment of Follicle Parameters
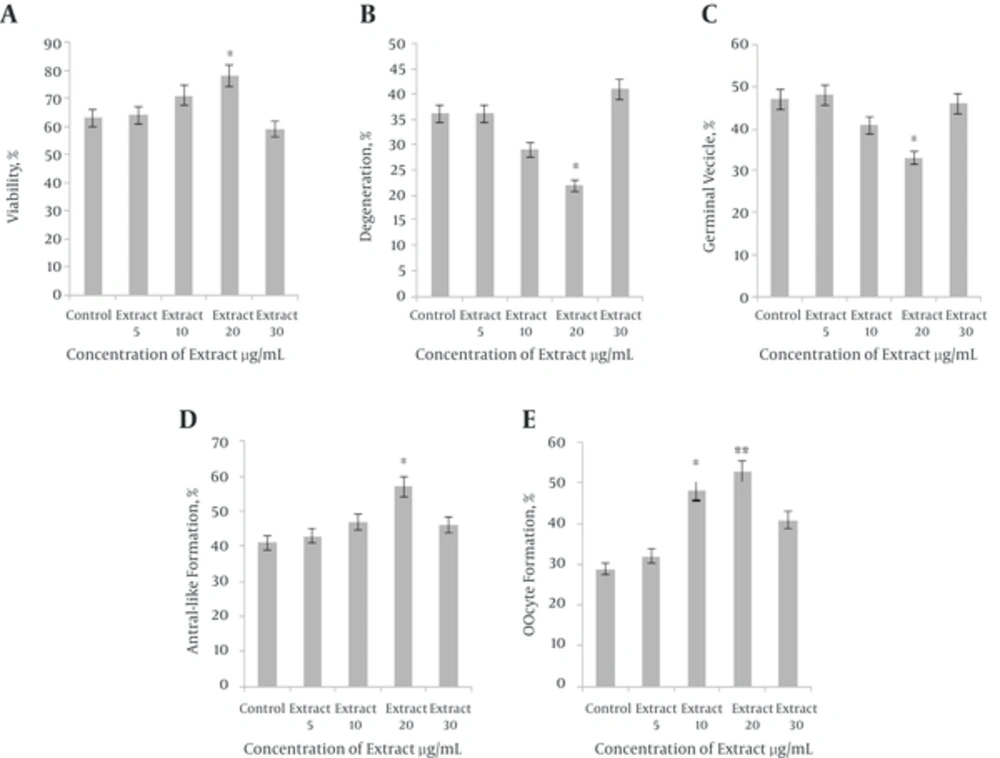
The follicle morphology is evaluated and the follicular development stage is classified as follicular, diffuse or antral in the in vitro preantral follicle culture. The percentage of arrested oocytes at GV stage in the control group was significantly (P < 0.05) higher than treated group with 20 µg/mL sea cucumber extract. Oocytes treated with 20 µg/mL showed the minimum percentage of GV stage compared with control, 5, 10 and 30 µg/mL sea cucumber extract (P < 0.05); however, no significant difference observed at percentage of GV stage between control and treatment groups. Significant differences were found between treated (10, 20 μg/mL) and control groups in follicle diameter (P < 0.05). The percentage of antral like cavity formation in treated group (20 μg/mL) was significantly (P < 0.05) higher than control and treatment groups. There was significant (P < 0.01) difference between treated group with 20 μg/mL extract and the control in percentage of maturated oocytes to metaphase II (MII) stage. In addition, significant differences were found between treated group with 10 μg/mL sea cucumber extract and control group in maturated oocytes percentage to metaphase II (MII) stage (P < 0.05). The oocytes treated with 20 µg/mL extract achieved the maximum percentage of MII stage and antrum formation compared to control group. There were no significant differences in the percentage of MII stage in treated groups (5, 30 µg/mL) and control group (P > 0.05) (Table 1) (Figures 1 and 2).
| Different concentration of sea cucumber, µg/mL | Total | Viability % | Degeneration % | GV % | Antrum % | Maturation % |
|---|---|---|---|---|---|---|
| 0 | 123 | 63 ± 4.58 | 36 ± 5.56 | 47 ± 4.00 | 41 ± 4.35 | 29 ± 3.66 |
| 5 | 78 | 64 ± 5.00 | 36 ± 5.56 | 48 ± 5.56 | 43 ± 6.00 | 32 ± 5.56 |
| 10 | 73 | 71 ± 7.21 | 29 ± 5.56 | 41 ± 5.56 | 47 ± 5.56 | 48 ± 8.54 |
| 20 | 81 | 78 ± 5.56 | 22 ± 4.58 | 33 ± 2.51 | 57 ± 2.64 | 53 ± 4.00 |
| 30 | 83 | 59 ± 3.60 | 41 ± 3.60 | 46 ± 4.35 | 46 ± 4.35 | 41 ± 5.29 |
aData are expressed as mean ± SD.
bPercentage of viability, oocytes arrested at germinal vesicle (GV), oocytes at antral-like cavity stages and metaphase II oocytes (MII) stage; All experiments were repeated eight times.
4.2. Determination of Antioxidant Capacity
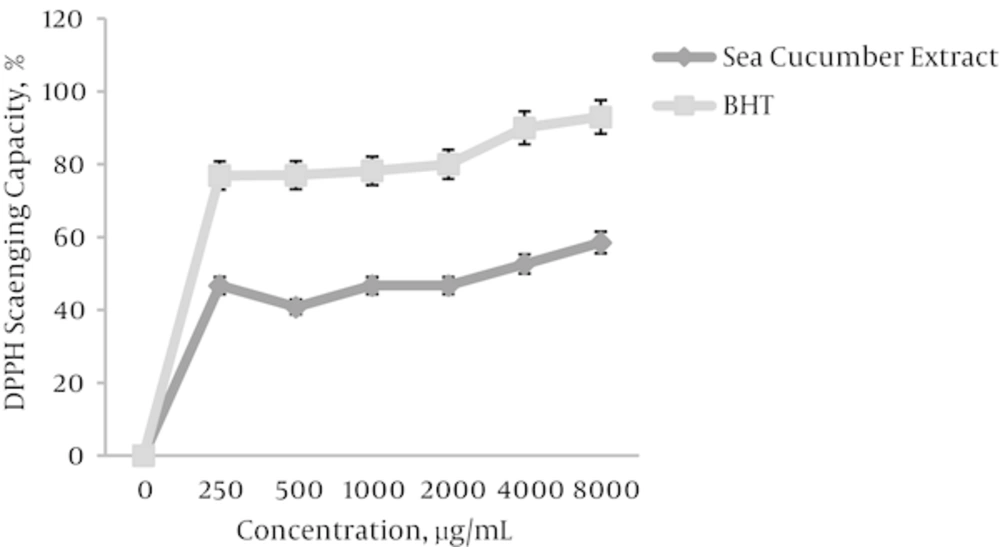
The anti-oxidative potential of sea cucumber alcoholic extract was evaluated using the DPPH free radical scavenging assay. Figure 3 showed that sea cucumber dose dependently exerted DPPH scavenging effect with 51.9 % scavenging capacity in a concentration of 1 mg/mL. Therefore, depending on concentration, the methanol extract exhibited a moderate DPPH-scavenging activity as compared to the BHT as a standard antioxidant compound.
4.3. Evaluating the Expression of TNF-α by Flow Cytometry
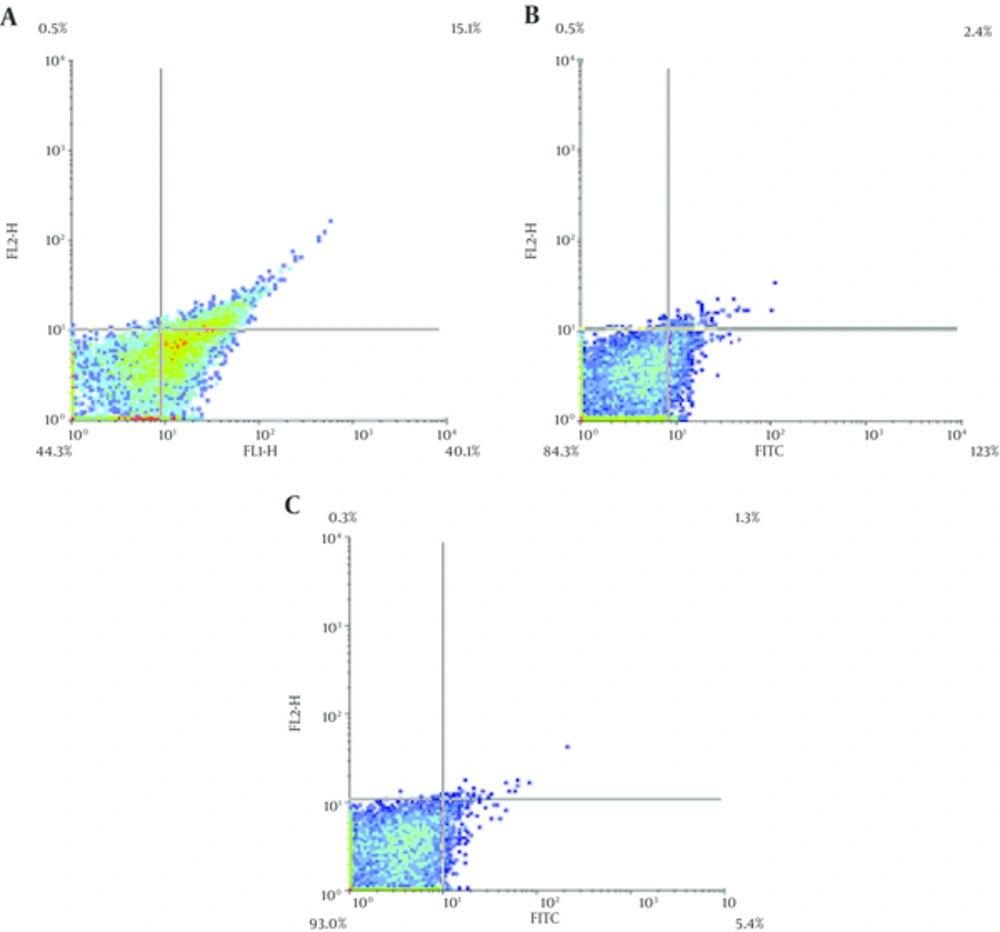
We examined whether sea cucumber extract can exert its promoting effect via inhibition of TNF-α or not. Flow cytometry diagrams revealed that sea cucumber extract significantly down regulated TNF-α expression level in a dose-dependent manner. TNF-α level in control group was 55.2% and in sea cucumber treated group (10 and 20 μg/mL) was 14.7%, 6.7% respectively. These results indicated that sea cucumber extract in concentrations of 10 and 20 μg significantly reduced TNF-α which is related with ovulation stage (Figure 4).
5. Discussion
To our knowledge, this study is the first report to prove the advantageous effect of sea cucumber extract as supplementation micro-environment in IVM. In this study, the DPPH scavenging effect of sea cucumber extract proposed antioxidant capacity of this extract which conferred potential to counteract with stress oxidative. The reactive oxygen species (ROS) and free radical generation induced oxidative stress that possess a damaging effect on early in vitro embryo development [23].
Althunibat et al. [24] exhibited that sea cucumber various species reduced the levels DPPH and suggested that sea cucumber are proposed as potential sources of natural antioxidant which in accordance to our study, demonstrated antioxidant potential of sea cucumber species. Esmat et al. [25] reported that Red Sea (H. atra) possess physiologically active phenolic compounds with antioxidant capacity which afforded a potential hepato-protective activity against thioacetamide induced liver injury in a rat model. These findings in agreement with our results confirmed antioxidant potential of sea cucumbers which promoted pre antral follicle in vitro maturation.
On the other hand, the present study assessed the supplementation effects of sea cucumber methanol extract on the follicular development and IVM of mouse oocyte. The beneficial effect of natural extract on in vitro maturation has been demonstrated previously [26]. Our results showed that the addition of optimum concentration of sea cucumber methanol extract during IVM process enhanced the percentage of in vitro maturated oocyte to MII stage. This means that addition of an appropriate concentrations of sea cucumber methanol extract in maturation medium have beneficial effects on IVM of mouse oocyte. The oocyte treated with 20 µg/mL extract achieved the highest percentage of in vitro maturated oocyte to MII stage.
It was demonstrated that supplementation of green tea polyphenols (GTP) during maturation of bovine oocyte increased blastocyst formation [27]. Moreover, In vitro culture of mouse oocyte in maturation medium with saffron (Crocus sativus L.) extract increased maturation and fertilization rate of oocyte [26]. It has been shown that the optimum concentration of Papaver rhoeas extract (100 µg/mL) in mouse oocyte maturation medium improves the rate of oocyte maturation and subsequently enhanced the rate of embryo development [28]. Rajabi-Toustani et al. [16] showed that Papaver rhoeas extract enhanced maturation rate of follicls dependent on the extract concentration, so that P. rhoeas extract with an appropriate concentrations (50 µg/mL) in maturation medium elevated the sheep oocyte maturation rate, while the results of this investigation elucidated the beneficial effect of natural extracts such as sea cucumber as supplementation in IVM process. Further, it is suggested that these beneficial effects are possibly related to their polyphenols contents (such as flavonoids and anthocyanin) that are the major compounds in this plant which is considered as the main reason of administrating antioxidant activity [27, 29]. These investigations in consistent with our results implied that natural substances possessing antioxidant capacity may be proposed as an appropriate supplement for in vitro maturation.
TNF-α is a pro-inflammatory cytokine that induces cell death. It was previously demonstrated that TNF-α significantly inhibited FSH-induced follicular development and steroidogenesis, therefore, TNF-α expression level was utilized for evaluation of follicular development [30]. In this in vitro study, we indicated that TNF-α protein level significantly inhibited under treatment with sea cucumber extract which proved that sea cucumber extract improved follicular development and maturation.
In addition, the results of this study showed that supplementation of appropriate concentrations of sea cucumber extract (20 µg/mL) in maturation medium improve the mouse oocyte maturation rate. However, the higher rate of mice oocyte maturation indicated that the improving effect of sea cucumber on oocyte development was related with antioxidant potential and down regulation of TNF-α protein expression level as degenerative follicular factor.
Nabiuni et al. [22] indicated that supplementation of in vitro maturation medium with natural antioxidants and anti-inflammatory agents such as bee venom improved maturation rate of mice preantral follicles which is associated with down regulation of TNF- α factor as necrosis inflammatory factor. Meanwhile, our findings confirmed improvement of preantral follicles maturation rate attributed with reduction of TNF- α expression.
In conclusion, the results of this investigation proved that the addition of marine natural extracts to maturation medium which possess natural antioxidants was innocent and can be useful in this field possibly by minimum side effects of synthetic material. Accordingly, sea cucumber may be regarded as a valuable source of bioactive compound for drug discovery in biomedicine.