1. Background
Learning and memeory are corrolated process that during of them different data stored in brain and recalled when requered [1]. Multiple factors can affect on memory and learning that the role of ions like magnesium and zinc can be significant [2, 3]. Magnesium is the forht major cation in body and the secound major intracellular cation after potassium [4]. Magnesium ion deficiency has important effect on memory loss in people with Alzheimer's disease and increasing magnesium intake led to increase of memory in young mice and decrease of memory loss in old mice [4, 5]. Zinc is a necessary elemnt for brain and other body organs physiological function [6]. Zinc ions play important role in behaviors associated with memory, learning and processes associated with brain aging so that the deficiency of zinc ion in diet can lead to loss of memory in animal models [7, 8].
On the other hands both of these elements have effects on common receptors like NMDA glutamatergic receptor that has impotrant role on memory processes and long term potentiation (LTP) [9, 10].
According to the above studies, suggests that the role of these elemnts in some cases such as learning and long term memory can be examined and compared.
It is notable that elements like zinc can influence blood-brain-brarier (BBB) permeability, while magnesium has a little ability in crossing of BBB [2, 11]. Intravenous injection of magnesium lead to little increase in cerebrospinal fluid magnesium [4].
There fore peripheral useage of these elements, because of the restrictions in crossing or disruption of BBB, is fundamental point for the treatment of zinc and magnesium deficiency in central nervous system.
By development of nano medicine, usage of metals oxide nanoparticles in some models of animal behavior growth up quickly [12-20]. Application of these nanoparticles, because of their special properties, could reduce some limitations of conventional drugs like restriction to crossing of blood-brain-barrier [15].
Our previous studies have shown that nanoparticles of metals oxide such as ZnO and MgO can influence some of behavioural responses [16-20]. Acute and chronic usage of nano ZnO has anxiolytic and antinociception effects in male and female rats [12, 13, 15, 16]. Nano MgO reduced pain conception in mice and has anxiolytic effects in rats [17, 18]. We have shown that post-training injection of nano MgO can improve the passive avoidance memory in adult male mice while post-training injection of nano ZnO can cause memory loss in adult male rats [19, 20].
2. Objectives
Since the effect of the nano ZnO and MgO in pre-training administration on long term memory has not been studied complately, the aim of this study is comparison between pre-training injection of ZnO and MgO nano particles, on long term memory in animal model of passive avoidance learning.
3. Methods
3.1. Animals
In this experimental study were used adult male NMRI mice weighting 25 ± 3 g. Animals purchased from the animal house of the medical science department of the joundi shapor university (Ahvaz, Iran) and were kept in plexiglass cages in room with 12/12 hours dark/light cycle and a temperature of 23 ± 2°C with adequate access to food and water. Animals were divided in to groups: control group (receiving saline %0.9), groups receiving nano Zno and/or nano MgO in doses of 1, 2.5 and 5 mg/kg (i.p.).
Nano particles of MgO (Lolitech Co; Germany particle size < 50 nm) and nano ZnO (Lolitech Co; Germany particle size < 70 nm) dispersed in saline %0.9, by ultrasonic bath (S2600 Co; Iran) for 15 minutes. Memory was evaluated by step- down apparatus (ST-5500 Co; Iran) as a passive avoidance learning model, 30 minutes after IP injection of drugs. Number of animals in each group were 7 (N = 7).
3.2. Step-Down Apparatus
This device is a box with three dimensions (40 × 30 × 30 cm). Three of plexiglass walls have dark colour and front wall made from transparent plexiglass that in it animal behavoir is visible. Graid floor of box is made of stainless steel and wooden platform (2.5 × 7 × 27 cm) (as a safe platform) set in the center of the graid floor. In training time mouse was placed on wooden platform and the step- down latency (SDL) was recorded. When the mouse stepped down and placed all its paws on the grid floor, received electrical shock (15 volt) for 15 seconds. SDL was defined as the time taken by the mouse to step down from wooden platform with its entire paw to grid floor. Immediately after shock animal is removed from the box and one day after training, the test stage was performed on trained mice. In this stage mice was placed again on wooden platforms in the box and step down latency recorded. The maximum time of stay on a wooden platform in this stage was 300 seconds. Three and seven days after training , like the first day of test, step down latency recorded again for investigation of long term memory in mice [21].
3.3. Open Field Test
Open field test is used for investigatin the effect of drugs on locomotor activity of animals different tests. Open filed apparatus is a plexiglass box (30 × 30 × 30 cm Co; Iran) that placed on the wooden plateform that with 4 cross lines divided into nine parts. If the animal head’s and two front limbs crossed from one of these lines considered one number for animal. The number of crossed lines during a certain period of time appearance as animal locomotor activity. When performing test, for animals adaption with the open field environment each mouse placed for 10 minutes in the ccenter of apparatus freely, and then locomotor activity recorded during 5 minutes The number of crossing lines during 5 minutes with animal head’s and two front limbs was evaluated as the locomotor activity index.
3.4. Statistical Analysis
Data analyzed with Instat 3 software and all results were expressed as the mean ± SEM. ANOVA was used for multiple comparisons between groups and post hock Tukey test was performed. Differences with a P value of < 0.05 between experimental groups at each point were considered statistically significant.
In the present study, all procedures methods were carried out in accordance with institutional guidelines for animal care and use of laboratory animals, and approved by the department of biology of the Shahid Chamran University (Ahvaz, Iran)
4. Results
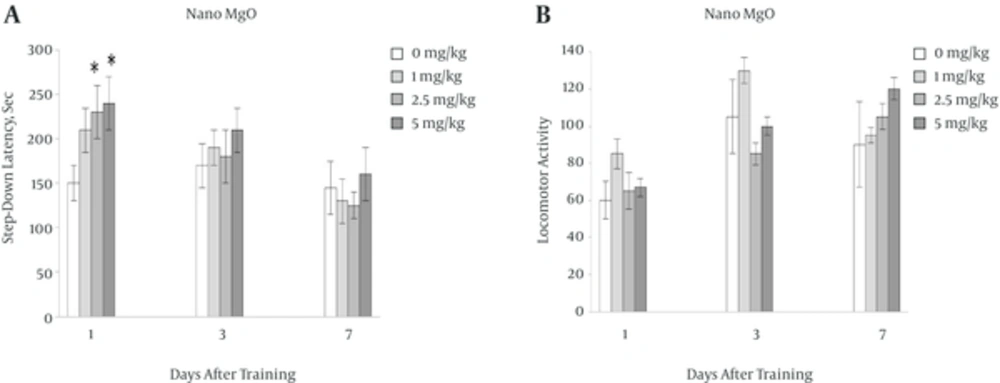
4.1. Effect of Pre-Training Injection of Nano MgO (1, 2.5, 5 mg/kg) on Passive Avoidance Memory and Locomotor Activity
Figure 1A showes that in nano MgO receiving groups (2.5, 5 mg/kg ), one day after training there was significant difference on step down latency level (SDL) in compared with control group (0 mg/kg)(*P < 0.05) . Nano MgO (5 mgkg) has tendency to increase memory at 3 and 7 days after traning but it was not significant.
Also Figure 1B shows that there were no significant difference in locomotor activity between all groups.
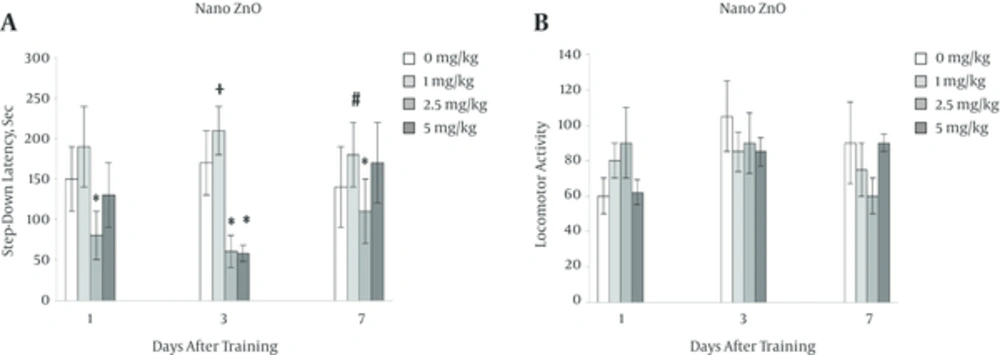
4.2. Effect of Pre-Training Injection of Nano ZnO (1, 2.5, 5 mg/kg) on Passive Avoidance Memory and Locomotor Activity
Results on Figure 2A showes that nano ZnO 2.5mg/kg significantly reduced memory at 1, 3 and 7 (*P < 0.05) days after training with decrement of step down latency (SDL). Also nano ZnO 5 mg/kg has tendency to reduce memory one day after training and significantly reduced memory three days after training (*P < 0.05). Figure 2B also shows that nano ZnO in all days after training has no effect on locomotor activity of mice.
5. Discussion
In the present study it was shown that, pre-training injection of MgO nano particles could improve memory in dose dependent manner only one day after injection and these doses could not change locomotor activity in every days after training (Figure 1A and B).
On the other hands nano ZnO 2.5 and 5 mg/kg (in a weaker level) significantly led to decrease of memeory in animals without any changes in locomotor activity of them (Figure 1A and 1B).
Our previous studies have shown that post-training administration of nano MgO can improve memory in mice [19]. Also pre-training injection of nano ZnO can cause memory loss in rats [20].
NMDA receptor is a glutamate gated channel in central nervous system that has important role in learning, memory and in long-term potentiation (LTP) mechanism [22].
Synaptic plasticity of LTP is a ability that regulates synaptic transmition performance with regulation of the NMDA receptor numbers and some changes in it’s subunits [23]. NMDA receptor is as the main target receptor in magnesium and zinc ions performance pathways [8-10, 24-26].
Magnesium acts as a noncompetetive antagonist for NMDA receptors [10] and has a certain place in cation channel of NMDA receptor that blocked it in resting potential [4]. Increasing of magnesium in brain, enhancing block and down regulating of NMDA receptor [26, 27].
It is notable that this receptor inhibited with magnesium just in short time of cellular potentional, resting potentional, and with depolarization of post sinaptic cell this inhibitation can be removed quickly [4].
Increase of brain magnesium can lead to increas in selective expression of some subunits of NMDA receptor [22, 26] also induction of LTP in synaps of perforant - dentate gyrus pathway and synaps of Shaffer clusters in CA1 area is depend on NMDA glutamateric receptors [26-28]. In CA1 area, NMDA receptor has an important role in regulation of synaptic plasticity, learning and memeory process [1, 28]. So magnesium can lead to increase of NMDA receptor activity, induction of LTP, increase of synaptic plasticity and improve of learning and memeory.
Up regulation in expression of NMDA receptor subunits is a homeostasis mechanism that not just returns activity of this receptor, that was reduced during resting potential, to initial level also causes more flow through this receptor and compensates it’s activity reduction [4].
In this experiment nano MgO has a tendency to improve memory only 24 hours after training and the next days statistically is not significant. It seems that despite the small size of MgO nanoparticles and it’s easier crossing through blood-brain-barier [14]. Acute administration of MgO nanoparticle has limited its improving effects and must be administered chronicaly for better function. Also toxical effects of nano MgO are as another inhibiting factors that prevent the reinforcing effects of it [29] that finding of these possible effects need to more studies.
On the other hands zinc ion affects NMDA receptor by two ways: noncompetetive voltage-dependent inhibition (allosteric) that reduces the frequency of channel valves opening and voltage-dependent inhibition that closes open channel by zinc ion [24].
Zinc inhibits NMDA receptor with high tendency at the 5 - 80 nanomolar concentration, voltage dependent inhibition with low tendency at the 45 - 79 micromolar concentration and inhibits receptor in voltage-independent manner with average tendency at the 1.6 - 9.5 micromolar concentration [24].
Dispaite of the magnesium, that act as a NMDA blocker, zinc ion make a noncompetetive- volatge-independent inhibition that probably reduces the frequency of channel valves opening [24, 30].
It seems that pre-training injection of nano ZnO probably increase zinc ions in glutamatergic synapse pathways and occures excessive inhibition of NMDA receptors, that ultimately reduces the incidence of LTP and reduces memory. Make changes in BBB permeability by zinc ions is other possible way.
In this experiment nano MgO had no long effect on memory while the effect of nano ZnO was longer than MgO. It seems that despite the small size of nanoparticles and easier crossing through blood-brain-barier, preservation of these particles in body may be related to the difference between their small size and or their toxicity because nano ZnO has been considered as one of the most toxic nanoparticles [14, 29].
5.1. Conclusion
Our findings show that pre-training injection of magnesium and zinc released from ZnO and MgO nano particles have different effects on passive avoidance memory process and this despite the fact that both of Zn and Mg were introduced as NMDA receptor, an impotrant receptor in memory process, blocker. On the other hands the difference in time of their effects maybe related to various toxicity and preservation of these nanoparticles in body. Evaluation of these probably reasons and the others ways need to more investigation.