1. Background
Lactic acid bacteria is a major part of normal human and animal gut microflora. It is also important for maintaining gastrointestinal health. As a naturally occurring probiotic, Bifidobacteria and Lactobacilli can modify the metabolic activities in the body by modulating immune system [1], producing antimicrobial agents such as hydrogen peroxide, antimicrobial peptides (bacteriocins), and organic acids such as acetic and lactic acids [2]. When used in adequate amounts in diet, they can synthesis vitamins (such as K and B), stabilize barrier functions and enhance the calcium and other mineral absorption on the gut. Different studies have also demonstrated positive effects of probiotic bacteria on bowel pH, intestinal regularity and the colonization resistance against pathogens [3, 4].
Prebiotics are Non-digestible food ingredients that allow beneficial changes. They may promote the survival or persistence of probiotic strains, enhance defense mechanisms of the host, increase resistance to various health disorders, and modify human gastrointestinal tract troubles [5]. Inulin type fructans fructooligosaccharides (FOS), and inulin are considered as prebiotics which are composed of β (2-1) linked fructosy l units with or without a terminal D-glucose at the reducing end. They have different degree of polymerization (DP) and may originate naturally as native components in many plants or derive through biochemical/enzymatic techniques [6-8].
A number of in vitro and in vivo studies have confirmed that inulin kind fructans are fermented in to lactic and short-chain carboxylic acids. Functional activities of some commercial prebiotics like NutraFlora P-95 (DP = 2 - 4), Raftilose P95 (DP = 2 - 7), Inulin-S (DP = 2 - 60), Raftiline HP (DP > 23) and GOS (galactooligosaccharides) (DP = 2 - 4) were assessed in in vitro studies [6]. Banuelos et al. tested the capacity of two probiotic strains, Lactobacillus gasseri CECT5714 and Lactobacillus fermentum CECT5716, to use several b (2-1) fructan mixtures as carbon source in in vitro cultures [8].
Jerusalem artichoke (Helianthus tuberosus) and Chicory (Cichorium intybus), belong to Compositae family, are two plant species used in commercial production of prebiotics [9, 10].
Jerusalem artichoke tuber contains nearly 13% - 18 % carbohydrates, of which about 80 % are inulin type fructans, 10% - 13 % sucrose, 3.5% - 5 % reducing sugars, 10% - 17 % proteins [11] and 0.8% - 0.9 % important minerals including K, Ca, P, Fe, Zn, Mg, Na, Cu and Mn [12, 13], and traditionally cultivates as food and animal feed [14].
In Jerusalem artichoke tubers the DP of fructans is rather low [15] and mainly depends on the plant source, variety, climate conditions and date of harvest [16, 17].
In the present study, the prebiotic potential of poly-fructans extracted from native Jerusalem artichoke tubers on the survivability and activity of B. bifidum and E. coli was investigated in "in vitro" conditions, and compared with prebiotic effects of HP-inulin.
2. Methods
2.1. Bacterial Strains
Two strains of pure commercial cultures used were Bifidobacterium bifidumPTCC1644 and Escherichia coli PTCC 1330. Both of them obtained from Persian type culture collection.
2.2. Media Preparation and Growth Conditions
The standard prebiotic of HP-inulin purchased from Orafti (Tienen, Belgium).The fructooligosaccharides of Jerusalem artichoke tubers (JA-Fr) were extracted according to Milani et al. [18]. B. bifidum growth media were MRS broth (DeMan-Rogosa-Sharpe Broth) supplemented with L-cysteine hydrochloride (0.5 g/L), sodium thioglycolate (0.2 g/L), and CaCl2.2H2O (0.1 g/L). TSB broth (Trypticase Soy Broth) was used for propagation of E. coli. Both media and all other chemicals purchased from Merck (Darmstadt, Germany). B. bifidum growth was anaerobically (Oxoid Anaerobic System with Gas Pak). The fructooligosaccharides free media were used as the control and the basal media which were sterilized by autoclaving at 121°C for 15 minutes. In the case of modified MRS broth, filter-sterilized L-cysteine hydrochloride was added to the autoclaved media. The inoculums were prepared from the standard strains stored in glycerol 12 % at -70°C using basal media. The filter-sterilized carbohydrates (JA-Fr and HP-inulin) added to the basal MRS broth and basal TSB broth to give final concentrations of 0.5 %, 1 %, 2 % and 3 % (w/v).3.3 Growth measurement.
In order to investigate the effects of JA-Fr on the growth and survivability of Bifidobacterium bifidum and Escherichia coli strains, the bacteria were cultivated overnight in the appropriated basal medium at 37°C. The activated cultures centrifuged for 15 minutes with 2500×g at 4°C (sigma centrifuge model 2 - 16 p), then the precipitate was washed twice with Phosphate-buffered saline (PBS) (0.1 M phosphate buffer pH = 7.4, 0.9 % saline), and the final pellet was suspended in PBS and diluted to about 106 cells/mL for B. bifidum and 109-1010 cells/mL for E. coli. The bacterial suspensions were inoculated at 1 % (v/v) concentration in to different testing media containing fructans. Then the cultures were incubated at 37°C for 24 hours. The turbidity at 620 nm of each culture was determined every 4 hours by subtracting A_620values of bacterial free medium from each test media. All measurements were repeated at least twice.
2.3. Growth Kinetic Parameters
Specific growth rate (µ) was calculated for each microorganism during its exponential growth phase by the formula:
Equation 1.

Where x and x0 are absorbance measured at time t and t0, respectively.
The generation time (tg) was calculated for each culture from the corresponding value of (µ) by the formula:

2.4. pH Changes
pH value was measured using Inolab pH meter model WTW (Inolab).
2.5. Statistical Analysis
The obtained results were analyzed by MINITAB 14 and MSTATC software and significant differences between groups were determined by the Duncan’s multiple range test. Differences were considered significant at P < 0.05.
3. Results
3.1. Effects of Fructans on Microbial Populations
Bifidobacterium bifidum and Escherichia coli are the strains associated with human and animal digestive systems. In vitro experiments on the comparative fermentation of selected fructooligosaccharides showed that the JA-Fr was fermented by B. bifidumPTCC1644, and it had the potential to stimulate the growth of bacteria (Table 1).
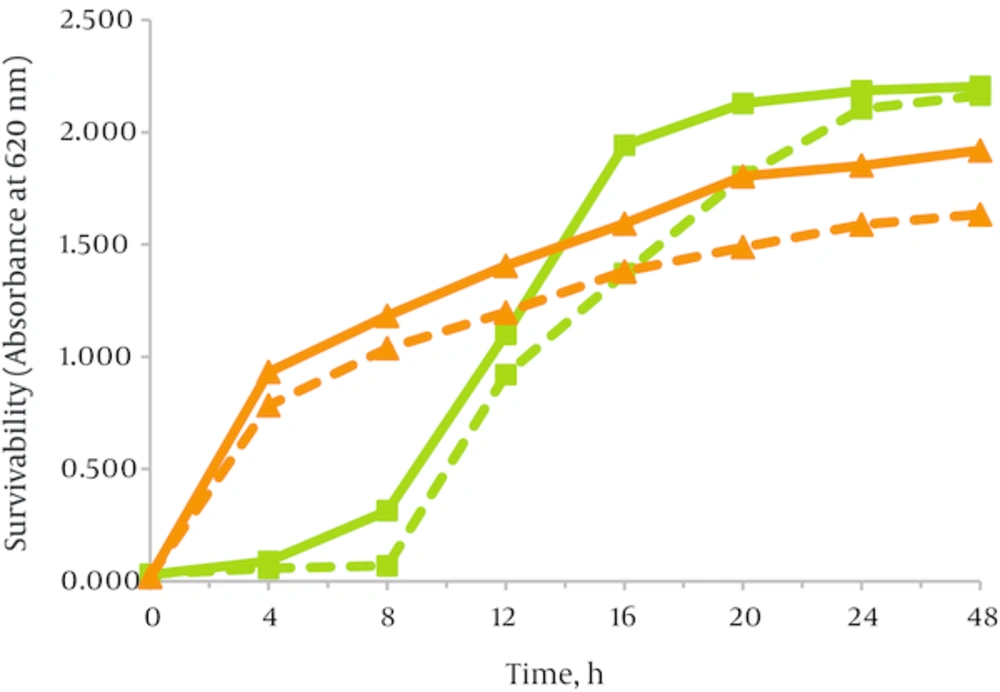
As shown in Table 2, supplementation with JA-Fr was found to have significantly better effect on survivability of the bacteria compared with that without JA-Fr (P < 0.05). We did not observed significant differences in the amount of cell growth as the concentration of JA-Fr rose to 3 % (w/v). Growth curve of B. bifidum showed that the bacteria grew rapidly after about 8 hours, with maximum growth observed at about 24 hours (Figure 1). Determined turbidities of the media containing JA-Fr were significantly (P < 0.05) higher than the ones containing HP-inulin, indicating that JA-Fr was more effective in modifying the growth behavior of Bifidobacterium bifidum PTCC1644.
| Bifidobacterium bifidum | |||
|---|---|---|---|
| Fructan in the growth media | Control | JA-Fr | HP-Inulin |
| Absorbance at 620 nm | 1.198 ± 0.06b | 1.237 ± 0.06a | 1.126 ± 0.08c |
| P-Value | 0.004 | 0.004 | 0.007 |
aThe data represent the results of a duplicate experiment.
| Bifidobacterium bifidum | |||||
|---|---|---|---|---|---|
| JA-Fr Concentration (w/v) | 0% | 0.5% | 1% | 2% | 3% |
| Survivability (Absorbance at 620nm) | 1.090 ± 0.003b | 1.239 ± 0.008a | 1.217 ± 0.004a | 1.266 ± 0.009a | 1.2230.008a |
| P-Value | 0.002 | 0.009 | 0.005 | 0.001 | 0.002 |
aDifferent letters mean statistically significant difference among the values of the same parameter, according to Duncan test (P < 0.05).
Specific rates of growth were determined for the media supplemented with 2 % (w/v) JA-Fr compared to the control and HP-inulin (Table 3). JA-Fr caused greater in vitro growth rate of Bifidobacterium than did the control and HP-inulin.
| Bifidobacterium bifidum | Escherichia coli | |
|---|---|---|
| Control | ||
| µ | 0.126 ± 0.007 | 0.902 ± 0.003 |
| tg | 5.50 ± 0.007 | 0.76 ± 0.003 |
| JA-Fr | ||
| µ | 0.142 ± 0.006* | 0.973 ± 0.004* |
| tg | 4.88 ± 0.006* | 0.71 ± 0.004* |
| HP-inulin | ||
| µ | 0.099 ± 0.001 | 0.905 ± 0.007 |
| tg | 6.97 ± 0.001 | 0.77 ± 0.007 |
aAll values for µ (h-1) and tg (h) are means from duplicate determination ± SD.
bSignificant differences at P < 0.05 confidence intervals (according to Duncan test) are shown as *.
To study the effects of different fructans on the growth of E. coli, the organism were cultured in the appropriate media supplemented with different concentrations of fructooligosaccharides at 37 °C. Results showed that they were fermented by the microbial flora (Table 4). Growth promotion of E. coli by JA-Fr has been dose dependent over the range 0.5 % to 3 % as evidenced by increased turbidity of the bacteria suspensions (Table 5), indicating that E. coli grew faster in the presence of these carbohydrates. Figure 2 shows the growth curves of E. coli strain cultured with both carbon sources. HP-inulin was found to be less effective on the viability of E. coli.
| Escherichia coli | |||
|---|---|---|---|
| Fructan in the growth media | Control | JA-Fr | HP-Inulin |
| Absorbance at 620 nm | 1.192 ± 0.08b | 1.233 ± 0.05a | 1.175 ± 0.09c |
| P-Value | 0.006 | 0.001 | 0.004 |
aThe data represent the results of a duplicate experiment.
| Escherichia coli | |||||
|---|---|---|---|---|---|
| JA-Fr Concentration (w/v) | 0% | 0.5% | 1% | 2% | 3% |
| Survivability (Absorbance at 620 nm) | 1.192 ± 0.002e | 1.272 ± 0.005d | 1.347 ± 0.007b | 1.392 ± 0.002a | 1.318 ± 0.009c |
| P-Value | 0.002 | 0.004 | 0.001 | 0.004 | 0.007 |
aDifferent letters mean statistically significant difference among the values of the same parameter, according to Duncan test (P < 0.05).
The doubling time (tg) of the strain grown in the presence of JA-Fr, HP-inulin and the control medium are compared in Table 3. Doubling time was used as a measure of the efficacy of various carbon sources in modulating growth rate. According to Table 3, tg in the medium containing JA-Fr was minimal.
3.2. pH Evaluation
The pH was recorded for the cultures with various carbohydrates. In accordance with growth stimulation, acid production by Bifidobacterium was also enhanced (P < 0.05) by the presence of JA-Fr in the media compared to the control (Table 6).
| Bifidobacterium bifidum | |||||
|---|---|---|---|---|---|
| JA-Fr Concentration (w/v) | 0% | 0.5% | 1% | 2% | 3% |
| pH | 4.86 ± 0.009a | 4.84 ± 0.005a,b | 4.82 ± 0.008b,c | 4.80 ± 0.006c | 4.75 ± 0.006d |
| P-Value | 0.003 | 0.001 | 0.003 | 0.002 | 0.005 |
aDifferent letters mean statistically significant difference among the values of the same parameter, according to Duncan test (P < 0.05).
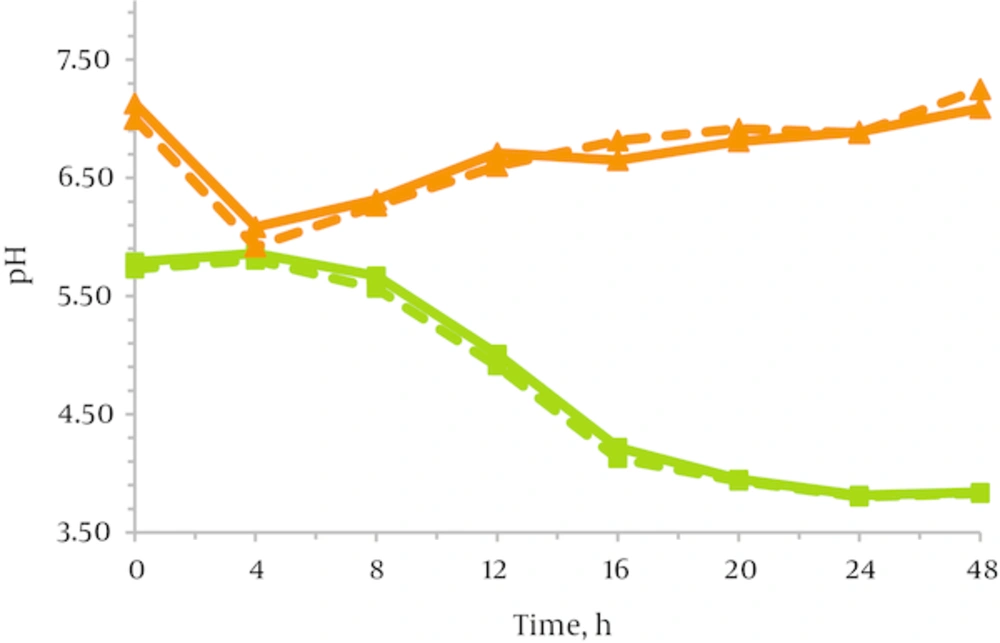
As shown in Figure 2, the pH continued to decline in a similar way in the presence of both JA-Fr and HP-inulin. After 24 hours of incubation, the pH dropped to 3.76 in JA-Fr-containing media, and to 3.87 in media with HP-inulin. The difference of pH values between two fructans was not significant (P < 0.05). Table 7 demonstrates the changes in TSB medium pH with different concentrations of JA-Fr which occur during fermentation.
| Escherichia coli | |||||
|---|---|---|---|---|---|
| JA-Fr Concentration (w/v) | 0% | 0.5% | 1% | 2% | 3% |
| pH | 6.78 ± 0.005b | 6.72 ± 0.009c | 6.86 ± 0.004a | 6.79 ± 0.008b | 6.50 ± 0.003d |
| P-Value | 0.003 | 0.005 | 0.003 | 0.003 | 0.002 |
aDifferent letters mean statistically significant difference among the values of the same parameter, according to Duncan test (P < 0.05).
pH has similar behavior in both media containing JA-Fr and HP-inulin. It was approximately 7.0 prior to inoculation, then there was a continued fall in medium pH over the first 4 hours and at this time, it began to rise (Figure 2). The difference between the pH values of two media was significant (P < 0.05).
4. Discussion
Our experiments on the supplementation with JA-Fr and HP-inulin showed that the JA-Fr had better effect on survivability of B. bifidumPTCC1644. Specific rate of growth determined for E. coli revealed that the efficacy of various carbon sources in stimulating bacterial growth were influenced by the concentration and DP of fructan chains in the media.
The ability of Bifidobacteria to utilize fructooligosaccharides has been reviewed in many studies and the lowering of culture pH as a result of short chain fatty acids production for certain bacterial species has often been used as an index of the fermentability of various carbohydrates in the culture [19]. Our results demonstrated that the viability of Bifidobacterium bifidumPTCC1644 and Escherichia coliPTCC 1330 in the media depend on the type and concentration of carbon source. Jerusalem artichoke fructooligosaccharides can be considered as a potential source for prebiotic production because it can provide the greater stability of probiotics and acid production.
Biedrzycka and Bielecka reported that the in vitro consumption of inulin by Bifidobacteria depended to purity and degree of polymerization of fructo-oligomeric chains. Their research indicated that the majority of Bifidobacterium strains studied utilized short chain FOS and OF [20]. Watson et al. showed that Lactulose, maltodextrin, FOS, GOS and the GOS/inulin (9: 1) mixture stimulate the growth performance of Bifidobacteria (12 different species), while inulin and polydextrose appeared to be rather poor substrates for bifidobacterial growth [21]. Inconsistent findings, Vigsnas et al. demonstrated that B. adolescentis and B. longum are able to degrade linear arabino-oligosaccharides (DP 8), whereas B. breve is able only to hydrolyze FOS and B. bifidum is not able to degrade either FOS or AOS [22].
Wichienchot et al. used mixed oligosaccharides obtained from white-flesh dragon fruit (Pitaya) and a reference prebiotic (inulin) as carbon sources for the cultivation of B. bifidum NCIMB 702715. It was found that inulin had a greater effect on the bacterial growth compared to Pitaya oligosaccharides, although the difference was not significant [23]. In another study, Wang et al. showed that the numbers of Bifidobacterium bifidumATCC 29521 were greater than those in control medium (P < 0.05) when cultured in the medium supplemented with alginate oligosaccharides. This compound stimulated the growth of B. bifidum, more significantly in comparison with fructo-oligosaccharides (FOS) [24].
In general, the ability of coliforms to utilize prebiotic oligosaccharides has been contradictory. Several studies have reported that FOS can support growth of E. coli, Enterobacter and Salmonella [25, 26]. In contrast, others have indicated that E. coli is unable to utilize FOS [27]. Lopez-Molina et al. studied the utilization of chicory and Artichoke inulin (different DP) in mixed cultures of colonic bacteria and showed that growth of Escherichia coli and total anaerobes was slower but longer-lasting in the presence of both inulins compared to the control with glucose [28]. Van Laere et al. reported that arabino-oligosaccharides could support the growth of E. coli but FOS could not [29].
Our findings were implying that the degree of polymerization of fructans was an important factor that decides the accessibility of fructans to the bacteria. According to Biedrzycka and Bielecka, susceptibility of saccharides to fermentation mainly depends on water solubility, chemical structure and degree of polymerization, chain length, branched or linear structure and composition of monomer units [19]. Shetty et al. reported that in "in vitro" fermentation of inulin by human fecal bacteria, molecules with DP > 10 were fermented on the average half as quickly as molecules with DP < 10 [30]. The degree of polymerization of fructans from Helianthus tuberosus tubers is rather low [15] in comparison with HP-inulin and mainly depends on the variety, climate conditions and time of harvest [16, 17]. In body, the lower pH is believed to have additional effects because the production of these acids reduces intestinal pH and restricts or prohibits the growth of many pathogen and putrefactive bacteria. Also it increases mineral uptake [31]. In the case of Escherichia coli, casein is a principal nutrient in TSB medium; so the changes in pH curves are probably due to cells metabolism especially the deamination of amino acids during bacterial growth [19, 32].
4.1. Conclusions
Regarding to the concept of synbiotic which is a mixture of probiotics and prebiotics that synergistically enhance equilibrium of the gastrointestinal microflora, finding new natural resources containing various prebiotic components could be an appropriate way to develop food industry and improve host health. Our results revealed that the survival and metabolic activity of Bifidobacterium bifidum PTCC1644 and Escherichia coli PTCC 1330 in the media depend on the type and concentration of carbon source. Jerusalem artichoke fructooligosaccharides can provide the greater stability of probiotics and acid production, so it can be considered as a potential source of high-yielding oligosaccharide for commercial prebiotic production; however further investigations are needed with other probiotic strains and in in vivo conditions to optimize the fructans concentration and bacterial growth.