1. Background
The use of medicinal plants is rapidly increasing in today’s society [1]. In United States between 1990 and 1997 found that about 38% of the population was used medicinal plants [2] and it was found that medicinal plants consumers are mostly women [3]. Women during pregnancy may use these herbs as natural and safe substances [4]. Over the past few decades, researchers have focused on drug discovery from herbal medicines or botanical sources, an important group of complementary and alternative medicine therapy. With a long history of herbal usage for the clinical management of a variety of diseases in indigenous cultures, the success rate of developing a new drug from herbal medicinal preparations should, in theory, be higher than that from chemical synthesis [5]. When taking herbs during pregnancy their unwanted side effects and teratogenicity should be considerered [6]. Elaeagnus angustifolia contains considerable quantities of flavonoid, terpenoids, Sytoastrol, carvacrol, alkaloidsand vitamins such as A, B, C and metals such as copper, zinc, iron, etc. [7, 8]. Medicinal properties of Elaeagnus angustifolia can refer to its antinociceptive, anti-inflammatory, antibacterial and antioxidant effects [9, 10]. Research has shown that Elaeagnus angustifolia compounds can easily cross the placental barrier and reach the fetus [11]. Talaei-Khozani et al. showed the effects of extracts of angustifolia on bone and cartilage in embryo mice limb bud in laboratory conditions and inside the body. In their experiments, pregnant mice were given 0.5, 5.0 or 50.0 mg/kg of the extract between days 8 and 18 of gestation. They showed the higher concentrations of angustifolia extracts had no effect on chondrogenesis or osteogenesis. However, pregnant mice received 50 mg/kg of this extract revealed a significant increase in their fetal femur and ossified zone length [12]. Since Elaeagnus angustifoliais possible to be used during pregnancy, it may has adverse effects on development of sensitive organ such as vision organ [13]. The aim of this study was to evaluate the effects of Elaeagnus angustifolia fruit aqueous extract on the histomorphometrical changes of retina in mouse embryo.
2. Objectives
Since, pregnant patients with joint pain and rheumatoid arthritis may used herbal extracts such Elaeagnus angustifolia. This study aimed to investigate the effect of aqueous extract of Elaeagnus angustifolia on fetal mice retina in Balb/C. In addition, this research consider to possible embryo toxic effects of prenatal exposure to Elaeagnus angustifolia by evaluating the histomophometrical and immunohistochemical parameters on the eye fetuses in the mouse embryos.
3. Methods
3.1. Preparation of Aqueous Extract
In this experimental study, all fruits were milled. Two hundred and fifty grams of milled fruit or its components were added to1000 mL water and boiled for 20 minutes, then filtered by a two-layer of fine mesh. The watery extract was concentrated on a boiling bath to the desired level, cooled and stored in a freezer at -20°C. The extract dissolved in distilled water to obtain a desired concentration before usage. To obtain moisture extract, two gram of final extract was placed in an oven in 60°C for 72 hours, and was then weighed and the weight loss was used as a moisture indicator. Thus, the final extract has 25% water.
3.2. Animals
After obtaining the approval of the institutional review board of our medical school, all experiments were carried out in accordance with the guidelines of the animal care and use ethics committee of Baqiyatallah University of medical sciences. Thirty adult Balb/C female mice weighting 33 ± 7 g, were maintained under standard laboratory conditions. Animals were housed in an environment of 21 ± 0.5°C with a relative humidity of 50 ± 10% and a 12 hours light-dark cycle. Food and water were always available. Estrous females were housed with Balb/C males for approximately 16 hours. The day of mating was considered day 0 of pregnancy. The pregnant mice were separated and were then randomly divided into experimental and control groups. Controls received tap water and aqueous extracts of Elaeagnus angustifolia at a dose of 500 mg/kg was administered orally (dissolved in drinking water) to the experimental groups from the first day to 18th day of pregnancy.
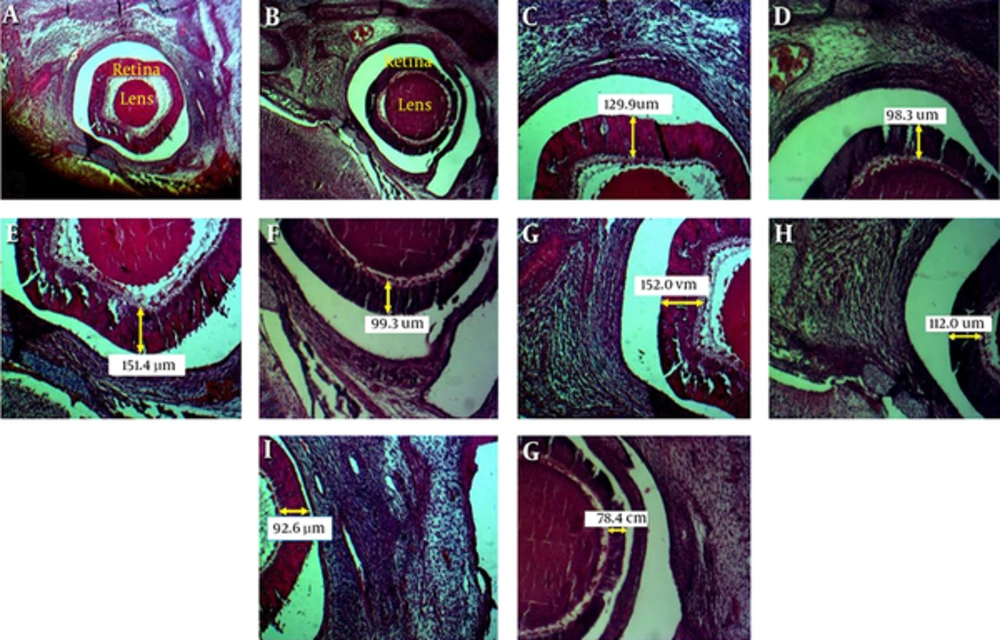
At 18th day of gestation, each pregnant mouse was killed from overdose of chloroform (Merk Co.). The uterine horns were exposed by laparotomy and their fetuses were removed from the uterus. In two groups fetuses were randomly selected from four to five litters. Fetal body weights and Crown-Rump lengths were measured and recorded. The fetuses were fixed in Bouin’s fluid overnight, followed by dehydration through graded ethanol solutions and were then embedded and their heads were sagittaly sectioned at 5 micrometer thickness through the midsagital plane. For each embryo, 20 sections are randomly selected at predetermined uniform intervals from all sections cut of the region of interest, using this manner for histomorphometric studies. Then the sections were stained with H and E (hematoxylin-eosin) technique.
3.3. Histomorphometric Studies
The thickness of the four areas of retinal layers was directly measured by using a Motic hardware and software system with a microscopic magnification of 40X objective lens. For each section, three measurements were taken randomly from non-adjacent points of the different thickness of retinal layers from the upper, lower, posterior and anterior parts of retina using the computer-assisted morphometric software (Motic software).
3.4. Cell Proliferation Assessment
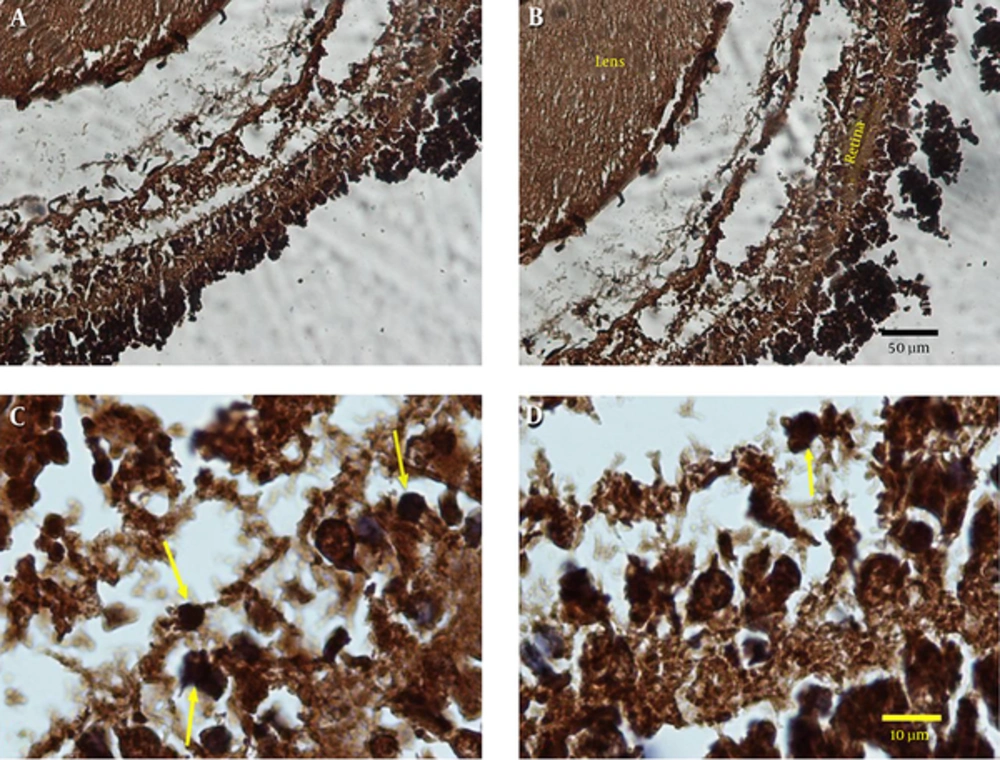
The standard method for assessment of cell proliferation in paraffin-embedded tissue sections is immunohistochemical analysis with the monoclonal antibody Ki-67, which reacts with a nuclear antigen in proliferating cells and then used avidin-biotin secondry antibody and stained with DAB chromogen.
The sections used for immunohistochemistry were dried at 37°C overnight, deparaffinized in xylene, and rehydrated through a series of graded ethyl alcohols. Endogenous peroxidase was inactivated by treatment with 3% H2O2 in 100% methanol for 5 minutes. Following a 5 minutes wash in phosphate-buffered saline (PBS, pH 7.4), microwave heat-induced antigen retrieval in 10 mmol/l citrate buffers, pH 6.0, was carried out. Non-specific staining was blocked by incubating the sections in 10% normal horse serum for 1 hour at room temperature. Afterwards, the sections were incubated with the Ki-67 primary antibody at room temperature for overnight in 2% normal horse serum in a humidified chamber. Sections were then washed in PBS and incubated overnight in either a donkey anti-mouse secondary antibody conjugated to biotin or a donkey anti-rabbit secondary antibody conjugated to biotin (both at 1: 1000 and Sigma USA) in 2% normal horse serum. Next, sections were washed in PBS and incubated with extravidin peroxidase (1: 1500, Sigma USA) in 2% normal horse serum for 4 hours. Sections were then incubated for 7 minutes with the chromogen DAB (diaminobenzidine) and glucose oxidase (both from Sigma, USA) for visualization of antibody binding. Finally, the sections were counterstained with hematoxylin and Cell proliferation as a percentage of ki-67 labeling cells in retina tissue in two groups were also evaluated.
3.5. Statistical Analysis
The Kolmogorov-Smirnov test was used to assess the normality of distribution of investigated parameters. All parameters in our study were distributed normally. All data were expressed as Mean ± SD. Differences were tested by SPSS 22 software package (SPSS Inc., Chicago, IL) using two-tailed t-test. The values P < 0.05 were considered statistically significant.
4. Results
4.1. Fetal Body Weights and Crown-Rump Lengths
The Crown-Rump length of the fetuses in experimental group significantly increased when compared to control group. Likewise, the mean body weight of the fetuses significantly increased in experimental group when compared to control group (P < 0.05).
The mean number of fetuses in experimental group decreased when compared to control group but the differences were not significant. The mean weight, diameter and thickness of placenta of the fetuses in experimental group significantly decreased compared to control group (P < 0.05) (Table 1).
| Parameters | Control | Experimental |
|---|---|---|
| Fetal weight, g | 1.15 ± 0.03 | 1.38 ± 1.35* |
| Crown-Rump length, mm | 19.96 ± 2.78 | 22.02 ± 3.47* |
| Number of fetuses | 5.53 ± 3.05 | 4.69 ± 2.64 |
| Weight of placenta, g | 0.18 ± 0.05 | 0.15 ± 0.03* |
| Diameter of placenta, mm | 8.06 ± 0.87 | 7.62 ± 0.53* |
| Thickness of placenta, mm | 2.78 ± 0.51 | 2.69 ± 0.37* |
a*Significantly different from control group (P < 0.05).
4.2. Histomorphometric Studies
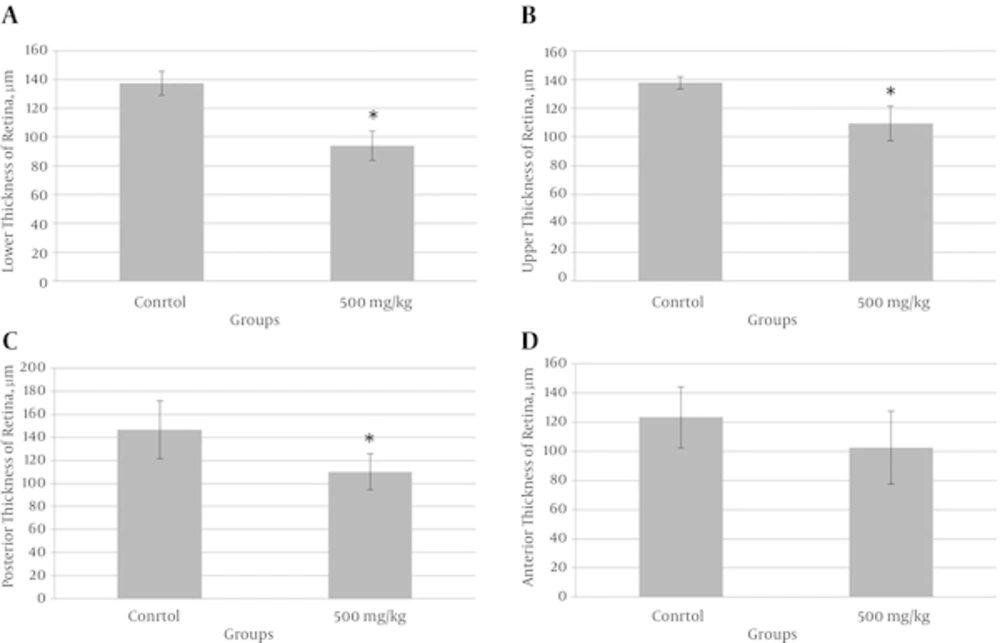
Histomorphometric evaluations showed that the mean of retinal thickness in superior, inferior and posterior parts significantly decreased in experimental group in comparison with the control group but the mean of retinal thickness in anterior part of experimental group has not any significance in comparison with control group (Figures 1 and 2).
The retinal thickness of inferior part (A), superior part (B) and posterior part (C) significantly reduced in experimental group compared to control group. However, retinal thickness of anterior part (D) in the experimental group was not different when compared to control group.*Significantly different from control group (P < 0.05).
4.3. Immunohistochemistry Results
Our immunostaining finding showed the number of Ki-67-positive cells in experimental group was decreased when compared to control group (Figure 3).
5. Discussion
Our study showed a significant increase in the weight and Crown-Rump length of embryos in the experimental group compared to controls. In agreement with our results are the findings of Talaei-Khozani about the effects of Elaeagnus angustifolia extract on bone and cartilage formation in mouse limb bud who reported pregnant mice received 50 mg/kg of this extract revealed a significant increase in their fetal femur and ossified zone length [12]. The influence of Elaeagnus angustifolia extract in the increase Crown-Rump length pattern of embryos is probably due to Flavonoid as ingredients of the extract. In mammals, flavonoid was observed in the diet of mother’s amniotic fluid, proving the placental transfer of this material as well as its anabolic effects on the embryos [14, 15]. Flavonoids also caused the increase in calcium compounds in diaphyseal bone tissue in femoral culture systems in rats [16]. It seems that the increase of Crown-Rump length in the fetuses exposed to Elaeagnus angustifolia extract are due to follow increase of calcium compounds which in the presence of Flavonoid are absorbed in a higher measure.
On the other hand, our study showed a significant reduction in weight, diameter and thickness of the placenta in the experimental group compared to controls. Our experimental findings are in agreement with the results of Anvari et al. in 2011, who studied on the effects of Zataria multiflora Boiss and reported the consumption of Zataria multiflora Boiss in the second week of pregnancy, caused a significant decrease in the size of tail embryo and in the mean of placental diameter [17]. It seems that carvacrol is one of the common combinations of Elaeagnus angustifolia and Zataria multiflora Boiss could produce these changes in the placenta in the experimental group [18, 19].
Our histomorphometrical results showed the decrease of retinal thickness in upper and lower parts in the experimental group compared to control group. It seems that presence of metals such as copper, zinc, etc. in Elaeagnus angustifolia extract may cause congenital malformations in the fetuses. Similarly, Parvari et al. revealed copper has a toxic effect on the central nervous system and can cause cellular changes in the embryonic nervous system and these changes could be seen in the forms of disorder in the cell positioning, increased intercellular spaces, cell swelling and nerve degeneration [20]. Similarly, teratogenic effects of Elaeagnus angustifolia extract has been shown on limb development [12]. It may be due to excessive amount of various vitamins A, C, E, and K. According to our finding, Rezaei et al. showed hyper vitamin A is a potent teratogen that causes developmental abnormalities in the central nervous system [21]. Finally, our immunohistochemical results indicated ratinal hypocellularity in experimental group compared to control group. This results revealed that Elaeagnus angustifolia extract reduced cell proliferation in fetal mouse retina.
In conclusion, the data suggest that prenatal exposure to Elaeagnus angustifolia extract has toxic effects on growth of retina of embryos as well as on the fetal and placental development.