1. Background
Myoma is one of the most frequent tumors in the women’s genital tract, especially in women of reproductive and menopause age [1]. Although the risk factors of myoma are not completely clear, some molecules such as estrogen, progesterone and the growth factors such as insulin like transforming growth factor β (TGF β) [2] and fibroblast growth factor (FGF) have been found related to the myoma [3]. Since hemostasis and coagulation system are responsible for pleiotropic function in cancer progression by forming the tumor microenvironment and metastatic niches through thrombin-dependent fibrin deposition and platelet activation, these two processes are considered as a probable pathogenic candidate for myoma [4]. These also have a significant role in the initiation and growth of tumors by inhibitory antibodies [4]. Tumorigenesis is associated with a hypercoagulant state and elevates the risk of thrombosis to four times [5]. New investigations have focused on the impact of inhibition of blood clotting proteins on cancer survival [6, 7]. EPCR and MTHFR are two genes with significant effects on thrombosis and some of their variations show correlation with increased formation of some tumors [8-10]. Protein C (PC) is a major component of the coagulation cascade. The interaction of PC-EPCR enhances the activation of PC about 20-fold. EPCR is a type I transmembarane receptor localized on the endothelial cells of large blood vessels. In addition to its APC (activated PC)-related functions, it plays an important role in thrombosis and inflammation too [11].
One of the most well-known variations in EPCR gene is rs867186 (AGT > GGT) which is associated with higher levels of Protein C and decreases the chance of thrombosis [12]. The role of two mutations in the MTHFR gene (C677T and A1298C) in thrombosis has been proven and their influence on the incidence of tumorigenesis in several kinds of tumor such as brain cancer, breast cancer, colorectal cancer, ovarian cancer, prostate cancer have been reported [13-15]. The substitution of C677T nucleotide of MTHFR gene, which reduces enzyme activity and results in increasing the level of homocysteine, can also be an important risk factor for venous thrombosis [16]. Hyperhomocyteinemia is another risk factor for thrombosis and the formation of blood clots [17]. The other polymorphism in the MTHFR gene, A1298C, results in the replacement of glutamic acid with alalnine. The 1298C allele clearly reduces MTHFR activity and may contribute to hyperhomocyteinemia.
However, several studies have reported the role of thrombosis in myoma tumors, which support the development of coagulation in myoma cases; there is no published research on the role of coagulation factors in myoma based on our knowledge [12, 18].
2. Objectives
The aim of this study was to investigate the role of three variations in two coagulation factors; EPCR and MTHFR, in the incidence of uterine myoma in Fars province.
3. Methods
3.1. Subjects
This case- control study was carried out in the hematology research centre, Shiraz University of medical Sciences from August 2014 to March 2015. Blood samples were collected from 73 women with uterine myoma with average age 38.4 ± 7 (mean ± SD) and 73 controls (50.5 ± 7) which gathered from Hazrate Zeynab hospital, Shiraz, Iran. All patients verified by laparoscopy technique. All the clinical procedure was approved by the hospital and the participants filled consent prior to their inclusion in the study.
3.2. Genotyping
The genomic DNA was extracted from blood samples contain Ethylenediaminetetraacetic acid (EDTA) with proteinase K method. The quantity of the extracted DNA was evaluated by spectrophotometry nanodrop and agarose gel electrophoresis. Genotyping was done using the thermal cycler BioRad T100 machine with the amplification refractory mutation system (ARMS-PCR) method. Primers were designed by primer 3 software and then evaluated by SNP check software for the probable existence of any single nucleotide polymorphism (SNP) in their length especially at the 3’ end. Primers and the length of the PCR products are listed in Table 1. The PCR condition for A1298C was as follows: Initial denaturation at 95°C for 3 minutes, followed by 30 cycles of; denaturation at 94°C for 15 seconds, annealing at 58.5°C for 30 seconds and extension at 72°C for 30 seconds. The amplification of C677T marker done in a condition as: primary denaturation at 95°C for 3 minutes, denaturation at 94°C for 35 seconds, annealing at 59.7°C for 45 seconds and extension at 72°C for 30 seconds, so that three last steps repeated for 35 cycles. Finally, the condition related to EPCR polymorphism was; primary denaturation at 95˚C for 3 minutes, 30 cycle repeats of denaturation at 94°C for 35 seconds, annealing at 65°C for 30 seconds and extension at 72°C for 30 seconds. Then one cycle of final extension step at 72°C for 5 minutes sets for all. Two reactions were done for each sample, each with an allele-specific primer. Totally, 5 primers were used, a pair of internal control hemoglobin subunit gamma 1 (HBG1) gene, a common primer and two allele specific primers. The PCR mix was prepared as follows with slight difference for each polymorphism: 5 µL of 1X PCR master mix, 10 pmoL of each allele specific primers, 4.5 pmoL of common primer, 2 pmoL of each HBG1 primers, 100 ng of genomic DNA plus dH2O up to the final volume of 15 µL. The products were then resolved on 2% agarose gel electrophoresis and stain with syber gold DNA safe stain dye, then visualized using a UV transilluminator. DNA molecular weight marker (Fermentas, 100 bp Ladder) was used to assess the size of the PCR products.
| Marker | Sequence 5′ to 3′ | PCR Product, bp | |
|---|---|---|---|
| 1 | MTHFR (1298) | Forward normal: TTGAAGGAGAAGGTGTCTGCGGTAGC | 781 |
| Forward mutant: TTGAAGGAGAAGGTGTCTGCGGTAGT | |||
| Reverse common: CAAGTGATGCCCATGTCGGTG | |||
| 2 | MTHFR (677) | Forward normal: AAGCTGCGTGATGATGAACTC | 226 |
| Forward mutant: AAGCTGCGTGATGATGAACTT | |||
| Reverse common: TTGGAAGGTGCAAGATCAGAG | |||
| 3 | EPCR | Forward normal: CACACCAGCAATGATGATACT | 243 |
| Forward mutant: CACACCAGCAATGATGATACG | |||
| Reverse common: TAGAGCAACAAGAGGCCCACAG | |||
| 4 | HBG1 | F: AACGGCTGACAAAAGAAGTCCTGG | 564 |
| R: TGCCAGGCACAGGGTCCTTCC |
Abbreviations: MTHFR, methylene-tetrahydrofolate reductase; EPCR, endothelial protein C receptor; HBG1, hemoglobin subunit gamma 1.
3.3. Statistical Analysis
Quantitative data were expressed as a mean± standard deviation (SD). Differences Categorical variables were presented using frequency counts and compared with a logistic regression test. Deviation from Hardy-Weinberg equilibrium was analyzed by a χ2 test. Odds ratio (OR) and 95% confidence interval (CI) were calculated as an estimate of risk. P < 0.05 was considered statistically significant. All statistical analyses were performed with SPSS 16.0 software.
4. Results
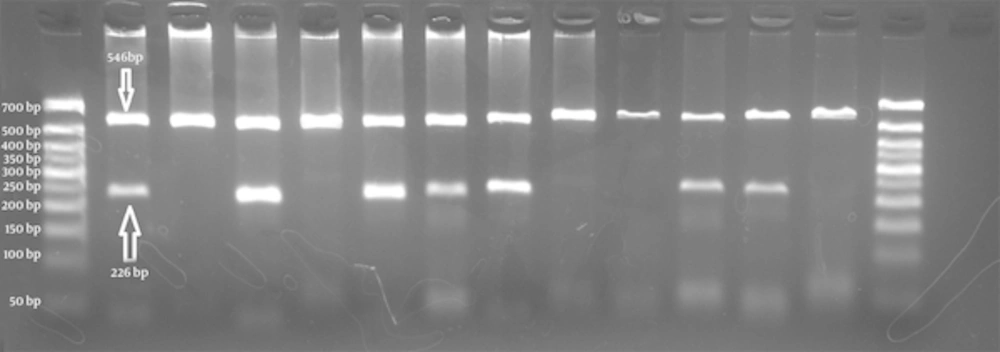
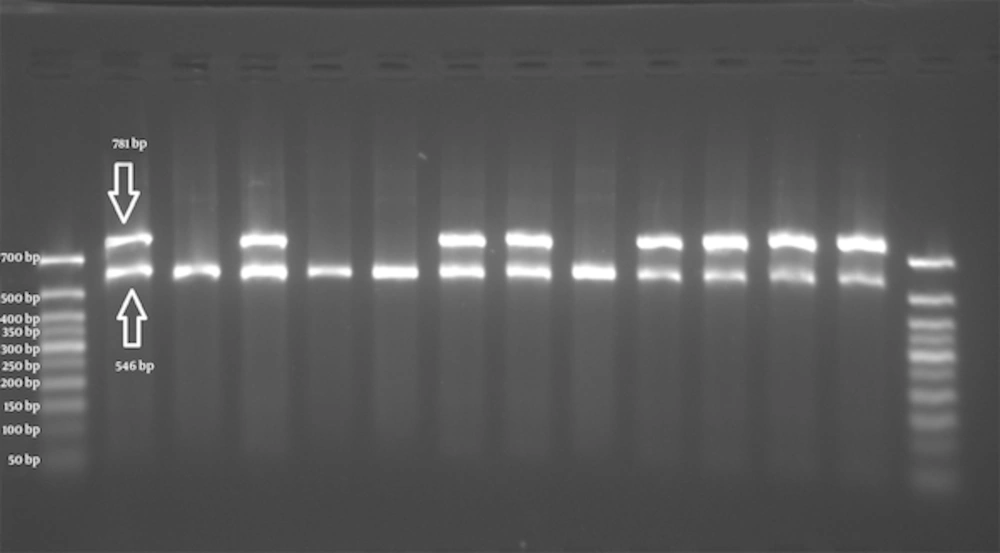
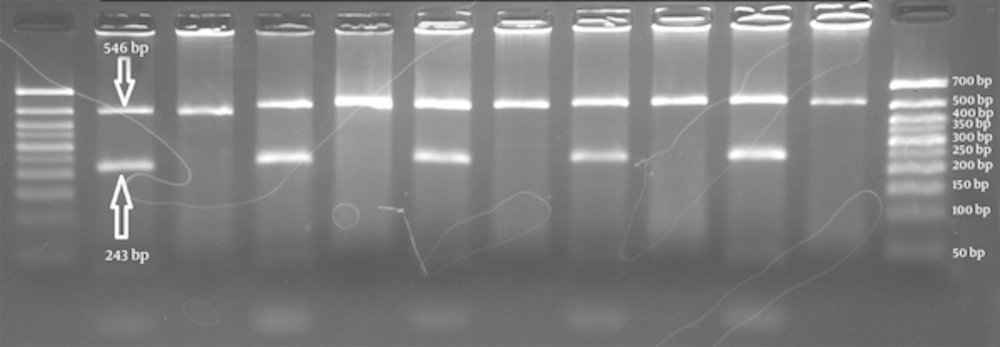
The schematic representations of ARMS-PCR for each polymorphic marker were shown in Figures 1 - 3. The total 73 individuals with uterine myoma tumors and 73 individuals as the control subjects were involved in this study.
The allelic and genotype frequencies of two polymorphic markers of MTHFR rs1801131, rs1801133 were presented in Tables 2 and 3 respectively.
First well in each case belongs to the primer for wild type allele C and the second well for mutant primer T (left to right). Wells 2/3, 4/5, 8/9 and 12/13 show homozygote CC genotype. Wells 6/7 is an indicator of heterozygous CT. Wells 10/11 are for the homozygote genotype TT. The 50bp size marker is loaded in first and last wells.
| A1298C Polymorphism | Controls (120), No. (%) | Patients (120), No. (%) | P Value | OR | 95% CI |
|---|---|---|---|---|---|
| Genotypes | |||||
| AA | 71 (97.3) | 63 (86.3) | - | 1 | reference |
| AC | 0 (0.0) | 8 (11) | 0.04 | 0.22 | 0.04 - 1.08 |
| CC | 2 (2.7) | 2 (2.7) | 0.99 | 0.0 | 0.0 |
| CC + AC | 2 (2.7) | 10 (13.7) | 0.01 | 5.6 | 1.18 - 26.6 |
| Alleles | |||||
| A | 134 (0.92) | 144 (0.98) | - | 1 | reference |
| C | 12 (0.08) | 2 (0.02) | 0.01 | 6.44 | 0.4- 29.3 |
Abbreviations: OR, odds ratio; 95%CI, confidence interval.
| C677T Polymorphism | Controls (120), No. (%) | Patients (120), No. (%) | P Value | OR | 95% CI |
|---|---|---|---|---|---|
| Genotype | |||||
| CC | 62 (84.9) | 59 (80.8) | - | 1 | reference |
| CT | 7 (9.6) | 9 (12.3) | 0.57 | 0.74 | 0.26 - 2.11 |
| TT | 4 (5.5) | 5 (6.8) | 0.69 | 0.76 | 0.19 - 2.97 |
| TT + CT | 11 (15.1) | 10 (13.7) | 0.51 | 0.75 | 0.31 - 1.77 |
| Allele | |||||
| C | 131 (0.9) | 127 (0.87) | - | 1 | reference |
| T | 15 (0.1) | 19 (0.13) | 0.46 | 0.76 | 0.37 - 1.57 |
Abbreviations: OR, odds ratio; 95%CI, confidence interval.
After analyzing MTHFR C677T mutation and determining of allele frequency in normal and uterine myoma cases, there was no statically significant difference between this mutation and risk of myoma was found (P > 0.05). The frequency of the TT genotype in patients and controls was 6.8% and 5.5% respectively. The relationship between the T allele carriers and myoma was also analyzed which resulted in no relationship. By genotyping of MTHFR A1298C, we found a significant association between heterozygote genotype and the risk of uterine myoma (P = 0.04). Also genotypes with at least one C allele (CC + CA vs. AA) showed a remarkable increase in risk of myoma (OR: 5.6, 95% CI: 1.18-26.6, P = 0.01). The C allele frequency was 0.02 in myoma patients compared with 0.08 in controls (OR: 6.44, 95%CI: 0.4-29.3, P = 0.01). In this study minor allele for rs86786 in the EPCR gene was absent in both patient and control groups. The genotype frequencies in both patients (df = 1, P > 0.05) and controls (df = 1, P > 0.05) in the studied SNPs were not significantly differ from those expected for populations in Hardy-Weinberg equilibrium.
5. Discussion
A statistically significant difference was observed between patients and the control group regarding the frequency of alleles C in the MTHFR (A1298C) gene (P = 0.01). Uterine myomas is the most prevalent tumor in women’s reproductive or even after menopause age [1]. Since tumorigenesis and thrombosis closely connected, involved factors in thrombosis could be considered as genetic markers for myoma tumor, and therapeutic targets.
In this study, we focused on the most effective variations in two thrombotic factors, EPCR (rs867186) and MTHFR (rs1801113, rs1801133), and their effects on myoma tumor incident for the first time. The association of MTHFR genetic variations; rs1801133, rs1801131; with several types of tumors such as gastric cancer, breast cancer, colorectal cancer, ovarian cancer and prostate cancer have been reported [13, 14, 19, 20], and correlation of rs867186 in EPCR gene with venous thromboembolism and homeostasis was previously demonstrated [21, 22].
The presence of the G allele makes EPCR more sensitive to cleavage and shades EPCR from endothelial cells [23]. The protein C anticoagulant pathway plays as a major regulator of thrombosis, the inflammatory response and endothelial cell apoptosis [24, 25]. The endothelial PC receptor (EPCR) which mainly expressed on the endothelial cells of large vessels is mediator for these processes [26]. The rs867186 polymorphism in the EPCR gene in the membrane spanning domain of EPCR resulting in variation in EPCR levels in serum between 56% and 87% [22, 27, 28]. The variation in the EPCR gene or plasma sEPCR levels affect the risk of thrombosis [27, 28].
MTHFR mutations could increase the risk of tumorigenesis in two ways: firstly, lower levels of MTHFR activity could be the cause of a decrease in the level of 5-MTHFR an increase in homocysteine level, which lead to venous thrombosis. Since S-adenosyl-homocysteine [28], a product of MTHFR gene, acts as an inhibitor of several methyl transferases: also the reduction of 5-MTHFR may lead to disruption of DNA methylation and changing in expression of genes and altering cell proliferation [17, 29].
Our results demonstrated no association between myoma tumor and C677T variant in our populations, although the frequency of the T allele was higher in myoma group. The presence of the C allele of A1298C associated with the increased risk of myoma. Our results showed that the presence of at least one C allele increases the chance of uterine myoma.
Although, based on our knowledge, there is no similar study on myoma, several controversial researches have been done on the role of these two variations of MTHFR gene and different type of tumor: Jakubowska study showed that MTHFR C677T was associated with an increased risk and 1298A/C polymorphism has a negative effect on incidence of breast and ovarian tumor [30]. Sharp found both polymorphisms in association with breast cancer [31]. Other studies on breast cancer [32], colorectal cancer [28], gastric cancer [33] and lung cancer [34] also did not find any correlation between the existence of the T allele of C677T polymorphism and increasing risk of tumorigenesis.
Our results did not show any association between rs867186 in EPCR gene and the risk of myoma incidence. Genotyping showed the minor allele of rs867186 variation was absent in our participants. However, there have been no published study in this area yet, but other studies on other tumors have been done and have shown EPCR stimulates cell migration in breast cancer and stop the metastasis of tumor cells in lung cancer [4].
In conclusion, we reported the first prospective study, investigating the association between three coagulation related variations and risk of myoma. This study revealed a significant association between A1298T variation and myoma incidence that could be considered for further investigations and its potential for diagnosis of having a chance of myoma. Although same results did not gain for two other variations, the more research elucidate the role of MTHFR variations in tumorigenesis and future studies in larger populations is proposed to reveal the actual role of MTHFR variations in myoma.