1. Background
Sickle cell anemia (SCA) is about the most common single-gene disorder in sub-Saharan Africa, it has been estimated to have a prevalence of 3% in Nigeria [1]. In this disorder, there is the presence of mostly hemoglobin S (Hb S) in the red blood cells (RBC), and none of hemoglobin A (Hb A). Hemoglobin S is formed following a point mutation in the β-globin gene which leads to the substitution of valine with glutamine at the position 6 of the hemoglobin β-globin chain [1]. This substitution leads to formation of Hb S, which has decreased solubility particularly at conditions of low oxygen tension. Insoluble Hb S results in increased blood viscosity due to vascular sludging and with increasing concentrations of deoxy-Hb S, polymerization occurs with subsequent alteration in the properties of the cell membrane and ultimately the shape of the red cell [2]. The deformed (sickle) red cells are destroyed prematurely (in as short as 20 days from production) by the cells of the reticulo-endothelial system, leading to a chronic hemolytic anemia state [3].
Trace elements are involved in a number of critical biochemical processes in humans, such as cellular respiration, DNA and RNA reproduction as well as maintenance of cell membrane integrity [4]. In particular, copper, zinc and selenium are involved in the scavenging of free radicals which are generated in a number of conditions, including during infective processes [4]. A myriad of symptoms of ill health coupled with predisposition to infections and even organ dysfunction have been documented in patients with trace element deficiency or intoxication [4, 5]. Organ dysfunction in particular could potentially modify both disease phenotype and blood counts in patients with SCA [6].
There are a number of reports in literature which evaluated serum micro nutrients levels in patients with SCA. These appeared to have a consensus that SCA subjects have lower serum levels of micro nutrients when compared with normal (hemoglobin AA) controls [7-10]. It has similarly been observed that changes in serum levels of a number of micro nutrients tend to induce secondary changes in blood counts in non SCA subjects [11, 12]. For this reason, copper deficiency is an important differential in patients presenting with recalcitrant anemia [12]. To the best knowledge of the authors, no study had evaluated the relationship between micro nutrient levels and blood counts in SCA patients, particularly those from the south-east region of Nigeria.
2. Objectives
This study was therefore aimed at evaluating the relationship between serum levels of some trace elements and blood counts in a population of steady state sickle cell anemia patients in south-east Nigeria.
3. Methods
This was a cross-sectional study that was conducted among adult SCA patients who regularly attended clinic at the Hematology outpatient clinic of the department of hematology and blood transfusion, Nnamdi Azikiwe university teaching hospital, Nnewi, Anambra State, South-east Nigeria. Diagnosis was confirmed by cellulose acetate hemoglobin electrophoresis, in alkaline pH and patients were recruited over a period of three months. The Nnamdi Azikiwe University Teaching Hospital is an urban tertiary health centre, located in Nnewi, the commercial hub of South-east Nigeria; it serves as a major referral centre for the host state of Anambra, as well as neighboring Enugu, Imo and Delta States.
Study approval was obtained from our institutional review board and involved 28 consecutive sickle cell anemia patients who came for routine follow-up visit in the clinic and satisfied the inclusion criteria. These included;
a. Adult subject (age ≥ 18 years).
b. Confirmed SCA by alkaline electrophoresis
c. Steady-state clinical condition, as previously defined by Akinola et al. [13].
None of the patient was on Hydroxyurea, iron chelation therapy or chronic transfusion programme at the point of recruitment.
Seven milliliters of blood were drawn from each participant, from which 3 mL was transferred into Na-EDTA containing bottles for hemoglobin electrophoresis (by cellulose acetate hemoglobin electrophoresis, in alkaline pH) and full blood count determination. The remaining 4 ml was dispensed into plain tubes for determination of serum trace element concentrations, using the atomic absorption spectrophotometry (AAS). Full blood count was done using hematology auto-analyzer (Sysmex (KN-21).
All data was analyzed using the statistical package for social science (SPSS) software (SPSS Inc., Chicago, IL, USA) version 20. Relationships between the mean values of trace elements and blood counts were explored using the Pearson’s linear regression for bivariate correlation and level of significance was set at P < 0.05.
4. Results
The study subjects had a mean age of 20.29 ± 10.05 years, including 19 males and 9 females (M: F = 2: 1). The mean serum levels of micro nutrients in this study were 0.60 ± 0.29 mg/L, 0.13 ± 0.07 mg/L, 8.76 ± 1.14 mg/L, 0.40 ± 0.14 mg/L respectively (for selenium, copper, magnesium and zinc). Correspondingly, the means of white cell count (WBC), mean corpuscular volume (MCV), mean corpuscular hemoglobin concentration (MCHC), platelet count and hemoglobin concentration were 13.37 ± 8.78 x 109/L, 80.83 ± 15.33 fL, 32.29 ± 1.33g/L, 412.59 ± 232.45 x 109/L and 10.85 ± 12.80 g/dL respectively.
The result of correlation analysis between the means of serum levels of selenium, magnesium, and zinc and the full blood count parameters in all 28 study subjects did not show any significant relationship between these micro nutrients and blood count parameters (P values all > 0.5).
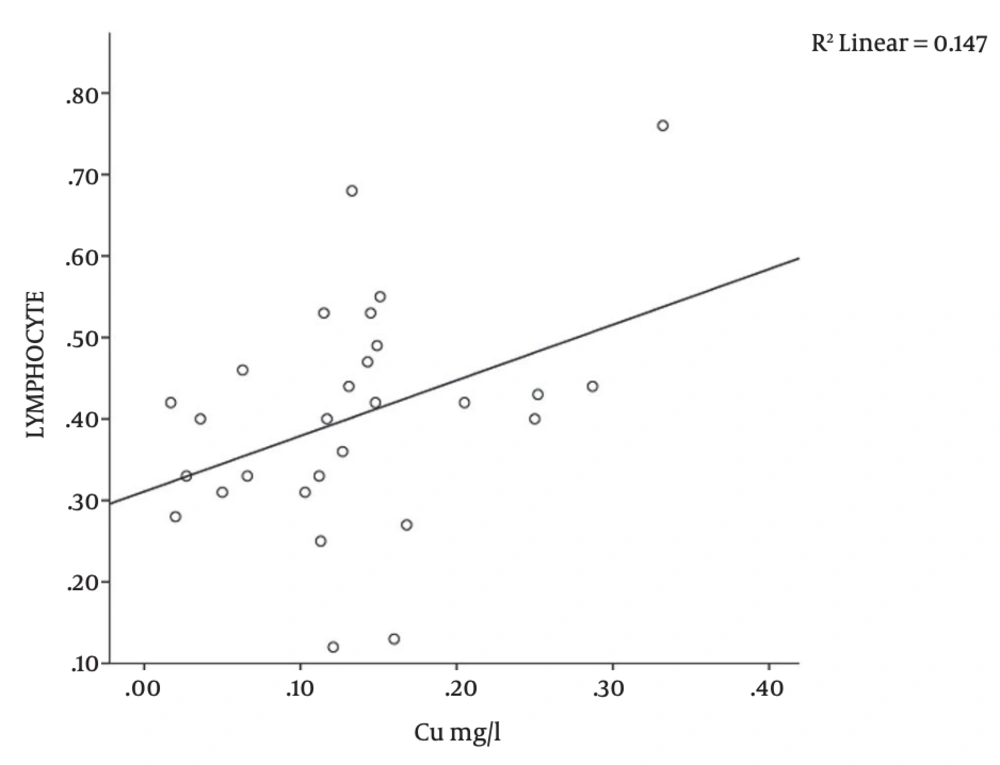
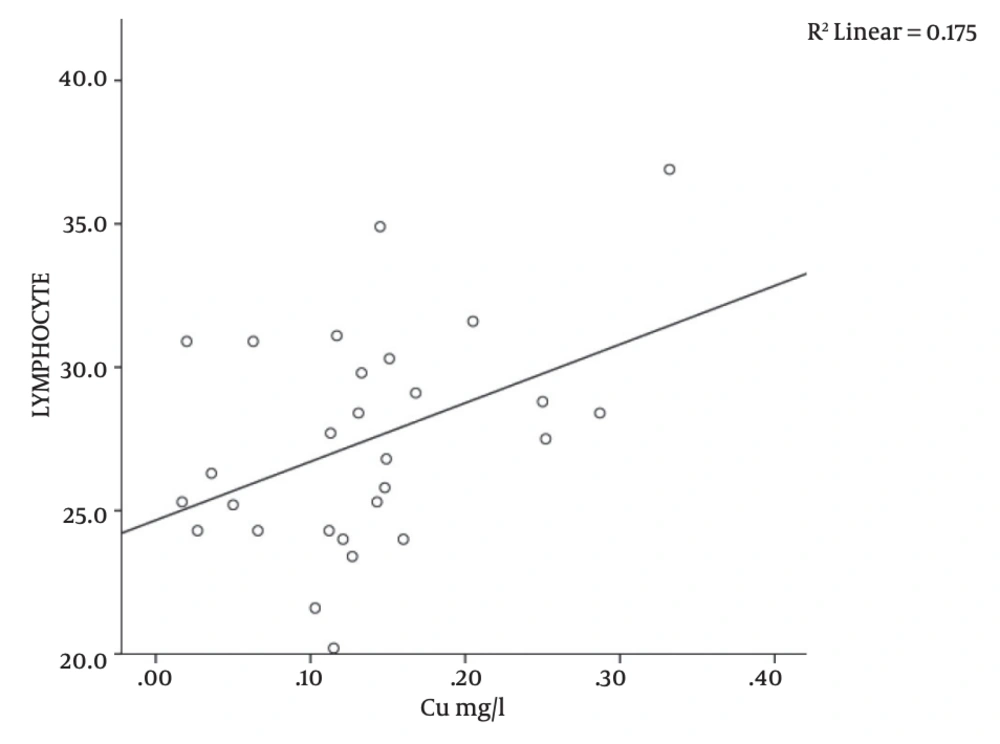
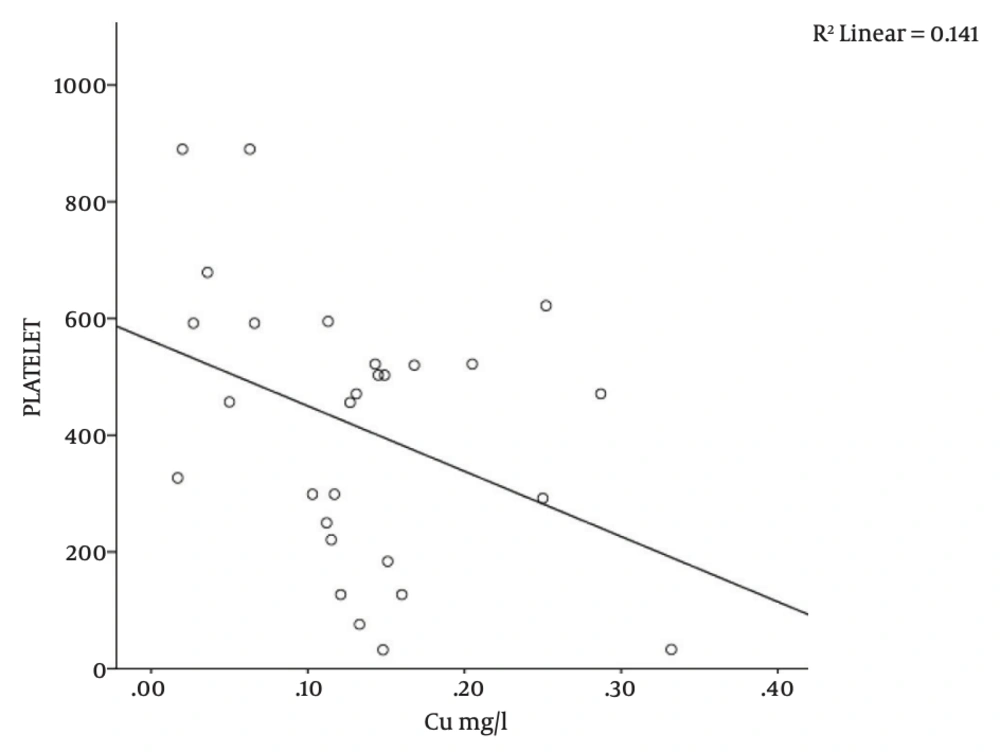
There was a significant correlation between serum copper level and platelet count (r = -0.376; P = 0.04), MCH (r = 0.418; P = 0.02), and lymphocyte counts (r = -0.383; P = 0.04) in the study subjects (Table 1 and Figures 1, 2, 3). There was no significant correlation between serum copper levels and other full blood count parameters (Table 1).
| Parameters. | Number (n) | Correlation Coefficient (r) | P Value |
|---|---|---|---|
| Copper vs. PCV | 28 | -0.188 | 0.33 |
| Copper vs. WBC | 28 | -0.190 | 0.33 |
| Copper vs. Neutrophil | 28 | 0.132 | 0.50 |
| Copper vs. Lymphocyte | 28 | 0.383 | 0.04a |
| Copper vs. Hb | 28 | 0.003 | 0.98 |
| Copper vs. MCV | 28 | -0.266 | 0.17 |
| Copper vs. MCHC | 28 | -0.037 | 0.85 |
| Copper vs. MCH | 28 | 0.418 | 0.02a |
| Copper vs. Platelets | 28 | -0.376 | 0.04a |
Correlation Between Serum Copper and Full Blood Count Parameters
5. Discussion
In this study, serum micro nutrient levels showed variable relationship with blood counts; while there were no significant correlation between serum levels of selenium, magnesium and zinc and blood counts, serum copper level was significantly correlated with platelet count, mean cell hemoglobin and lymphocyte count. Micro nutrients include a number of elements which are required in trace amounts to drive critical biochemical processes in the body. These come mainly from intestinal absorption, following oral ingestion [14]. As a result, their metabolism tends to interact either synergistically or antagonistically and this could be important in a number of disease states [15, 16]. This observation is demonstrated in nutritional anemia, in which the intestinal absorption of iron is up regulation along with that of other divalent metals, leading to their altered serum levels [14, 17]. It has been observed that micro nutrients such as zinc and copper impair iron absorption and could predispose to the development of anemia [16]. Infarct, Angelova et al. had concluded that clinical iron deficiency anemia is attributable to low serum levels of iron in only about 35% - 55% of cases, the rest is thought to be due to changes in serum levels of multiple trace elements [18]. Cao et al. determined the influence of iron status and trace element levels on growth and development among children in Shanghai, China and reported that trace elements were significantly related to children’s growth and development. The study advocated routine screening and dietary supplementation as potential interventions that could improve growth and development in this population [14]. Zinc has been found to play an important role in the regulation of erythropoiesis, control of iron metabolism and in the modulation of immunity to a number of infectious diseases. It is therefore not surprising that deficiency states of zinc has been associated with increased risk to infection as well as varying degrees of anemia [19, 20]. This study however did not show any significant correlation between serum micro nutrient levels and hematocrit (which could indicate the presence of anemia) in our patients.
Subjects with sickle cell anemia have been reported to have increased serum copper levels [7, 21]. Even though the clinical relevance of this elevation is not completely understood, a number of studies have associated this with decreased serum zinc levels [7, 21]. Prasad et al. reiterated this finding and noted that these patients in addition, have microcytosis and relative neutropaenia [8]. These abnormalities were reversed following copper supplementation. Other studies thereafter surmised that the finding of low circulating zinc and concomitant high circulating copper levels could be a marker of disease severity in patients with SCA (and even Alzheimer’s disease), since copper excess is thought to contribute to free radical formation and subsequent oxidant tissue damage [22, 23]. It was therefore suggested that the supplementation for copper and zinc in patients with SCA should be stringently balanced so as to forestall tissue toxicity from oxidant injury [4]. In this study, there was a significant negative correlation between serum copper level and platelet count (r = -0.376; P = 0.04, Table 1 and Figure 3). Even though the reason for this observation was not entirely clear, it is possible that there could be a preferential destruction of platelets, induced by copper, via oxidant cellular damage, thus accounting for the significant negative correlation observed in this study.
The MCH represents the amount of hemoglobin per red blood cell and is an important index in the evaluation of patients with anemia [24]. Studies have shown that intracellular copper could trigger red cell hemolysis (presumably through the generation of superoxide ions in the presence of sulfhydryl groups), as evident in Wilson’s disease [25]. The pre-existing chronic hemolytic anemia state of SCA could thus be potentially exacerbated by concurrent copper induced red cell lysis. Intravascular hemolysis, particularly when severe, could cause reticulocytosis; younger red blood cells are known to have higher hemoglobin content (higher MCH) than older red cells [26]. The above explanation may thus explain the finding of a significant positive correlation between serum copper levels and MCH (r = 0.418; P = 0.02, Table 1 and Figure 2) in this study. We did not observe any significant correlation between serum copper and mean cell volume (MCV) and neutrophil counts (r = -0.266; P = 0.17 and r = 0.132; P = 0.50, respectively, Table 1), this is in contrast to the report of Prasad et al. [8]. The reason for this discrepancy could not be explained from this study.
There was a significant positive correlation between serum copper levels and blood lymphocyte count (r = 0.383; P = 0.04, Table 1 and Figure 1). Turnlund et al. reported a significant increase in circulating lymphocytes in individuals on copper supplementation [27]. This may be due to the established role of copper in mediating normal lymphocyte maturation and regulating immune function [28]. These observations could explain the association between serum copper and blood lymphocytes in this study.
5.1. Limitations of the Study
This study is limited by the non availability of data on serum ferritin levels and the transfusion history of study subjects. Moreover, the small sample size could have influenced the power of the study to detect significant results.
5.2. Conclusion
Serum copper level in patients with SCA is significantly related to the MCH, platelet count, and blood lymphocytes counts. These relationships may be attributable to the roles of copper in inducing red cell hemolysis, in causing oxidant tissue damage and in enhancing the immune system, respectively.