1. Background
The etiology of DVT (deep venous thrombosis) is extremely diverse. The cause of DVT could be environmental and/or genetics. Family and twin studies indicated that genetic factors accounts for about 60% of the risk for DVT [1]. DVT is a commonly seen disease with a high morbidity, mortality and increase in miscarriages in pregnant women [2]. Age, drugs, surgery, smoking, cancer and immobilization are acquired risk factors and protein C and S deficiency and the presence of factor V Leiden are congenital risk factors for DVT [3]. Some results showed that a high plasma total homocysteine (tHcy) level might be a risk factor for development of DVT, but may not be an independent risk factor [3]. The human methylenetetrahydrofolate reductase (MTHFR) gene has been localized to chromosome 1p36.3 and is composed of 11 exons [4]. The C677T thermolabile polymorphism in the gene encoding 5, 10-MTHFR has consistently been associated with plasma tHcy levels. A point mutation, C to T substitution at the nucleotide 677, in the coding sequence of the gene for MTHFR is the most common enzyme defect associated with moderately-raised homocysteine concentrations, particularly in the presence of a suboptimal folate intake [5]. Two other polymorphisms, A1298C and T1317C, were identified in exon 7 of gene MTHFR. While variant T1317C is silent and does not alter the amino acid sequence, the A1298C polymorphism leads to the substitution of glutamic acid for alanine. Although still questionable, some studies showed that variant A1298C also reduces the normal activity of the enzyme involved in the breakdown of Hcy [6, 7]. There have been indications that even a slight excess of homocysteine in blood can result in increased risk of cardiovascular diseases [8].
MTHFR catalyzes the conversion of 5, 10-methylenetetrahydrofolate to 5-methyltetrahydrofolate, a co-substrate for homocysteine remethylation to methionine [4]. Elevated concentrations of total homocysteine have been related with an increased risk of arterial and venous thrombosis [9-11]. Mild hyperhomocysteinemia may result from a relative deficiency of folic acid and vitamin B12 and homozygosity for the common polymorphism in the MTHFR gene (C677T). Severe hyperhomocysteinemia is most often caused by the cystathionine β-synthase deficiency. Homocysteine is converted to methionine in normal adults. Methyl group and B12 are the co-factors in this reaction and methylfolate is the methyl donor.
Methylenetetrahydrofolate is converted to tetra-hydrofolate by enzyme MTHFR where methyl group is producd [12]. MTHFR thermolabile polymorphisms [MTHFR, C677T, ALA222VAL and MTHFR, A1298C, GLU429ALA] were investigated in other diseases such as occlusive artery disease [6] and cardiovascular disease [8]. DVT prophylaxis is effective when applied to cases that advantage the most from the significantly reduced thrombotic risk.
2. Objectives
In this study, we investigated the correlation between C677T and A1298C mutations on the MTHFR gene with total plasma homocysteine levels and deep venous thrombosis in pregnant women at risk of thrombosis and control group.
3. Patients and Methods
In this case-control study, informed consent was obtained from all groups. The present case control study included 120 pregnant women with risk of DVT and 100 pregnant women without risk of DVT as control group were admitted to hospitals in Tehran, Iran. The inclusion criteria for controls were: routine biochemical values within the normal range, nonsmokers and no history of renal failure, metabolic effects, cancer or cardiovascular pathology. Diagnosis of DVT was made by magnetic resonance imaging angiography, radioisotope venography and ultrasound based on previous studies done [13]. Study groups were similar in terms of geographic area.
3.1. Mutation Analysis
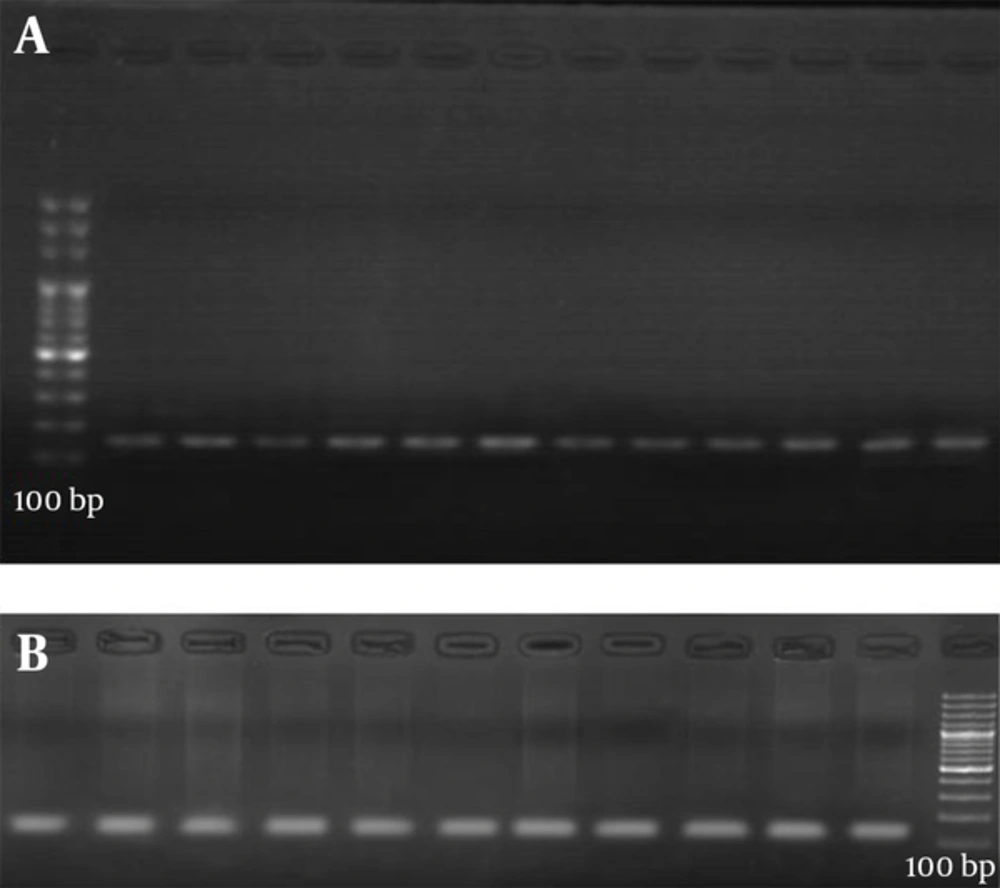
Genomic DNA was extracted by saturated salt standard method by a previously described method [14]. The C677T and A1298C mutations on the MTHFR gene were analyzed by PCR-RFLP. Primers are given in Table 1 accompanied with product values. The sequences of these primers are designed in previous studies [15, 16]. The primers for both polymorphisms are shown in Table 1. PCR for C677T mutation was carried out in a total volume of 40 μL containing 2.2 mmol/L MgCl2, 200 μmol/L of all 4 dNTPs, 0.5 μmol/L of each primer, 50 mmol/L KCl, 10 mmol/L tris-HCl (pH = 8.6), 10% glycerol, 1.5 U of AmpliTaq DNA polymerase (applied biosystems, Foster city, CA, USA), and 200 ng of template DNA. The reaction conditions were as follows: initial denaturation at 94°C for 4 minutes and 40 subsequent cycles of denaturation at 95°C for 30 seconds, annealing at 60°C for 30 seconds and final extension at 74°C for 30 seconds (Figure 1).
| Primer | Sequence | Annealing Temperature | Product Size, bp |
|---|---|---|---|
| 60 | 198 | ||
| F | 5'-TGAAGGAGAAGGTGTCTGCGGGA-3' | ||
| R | 5'-AGGACGGTGCGGTGAGAGTG-3' | ||
| 55 | 163 | ||
| F | 5'-CTTT GGGGAGCTGAA GGACTACTAC-3' | ||
| R | 5'-CACTTTGTGACCATTCCG GTTTG-3' |
PCR product (10 mL) was digested with 8 U HinfI (Gibco BRL, Paisley, Scotland) and 2 mL of buffer for HinfI for 12 hours at 37°C. The C677T mutation abolishes a HinfI restriction site. Digestion of the 198 bp fragment of the 677CC genotype results in two fragments of 175 and 23 bp, whereas the 677 TT genotype results in one fragment of 175 bp. DNA fragments were separated by electrophoresis on a 2% agarose gel and visualized with ethidium bromide. The second A1298C mutation was also analyzed the same as for the C677T mutation, plus 2 mmol MgCl2/L. The amplified fragment of 163 bp was digested with MboII (MBI fermentas, Vilna, Lithuania). The A1298C mutation abolishes an MboII restriction site.
Digestion of the 163-bp fragment of the 1298 AA genotype gives five fragments, of 56, 31, 30, 28 and 18 bp, whereas the 1298CC genotype results in four fragments, of 84, 31, 30 and 18 bp. The fragments were analyzed by 20% polyacrylamide gel electrophoresis and visualized with ethidium bromide. Because of its small size, the 23 bp, 84 bp, 31 bp, 30 bp, and 18 bp fragments were not seen on the gel. Homocysteine determination samples were taken after 12 hours fasting from the vein. After about 45 minutes the samples were centrifuged at 4200 × g for 8 minutes. Plasma fractions were aspirated and were stored at 20°C until analyzed. Total plasma homocysteine was measured by ELISA method using Axis Homocysteine kit (Axis-Shield, Dundee, Scotland). Statistical analyses were performed using SPSS-21 statistics software (SPSS Inc. Chicago, IL, USA). P value less than 0.05 were considered significant statistically. Allele frequencies were calculated by gene counting in patients and controls. The Hardy-Weinberg equilibrium was evaluated by the χ2 test for all the genotypes and category variables were studied with the χ2 test. Pearson P values, crude odds ratio (OR) and Wald’s 95% confidence interval (CI) were calculated to test the associations between MTHFR mutations and DVT.
4. Results
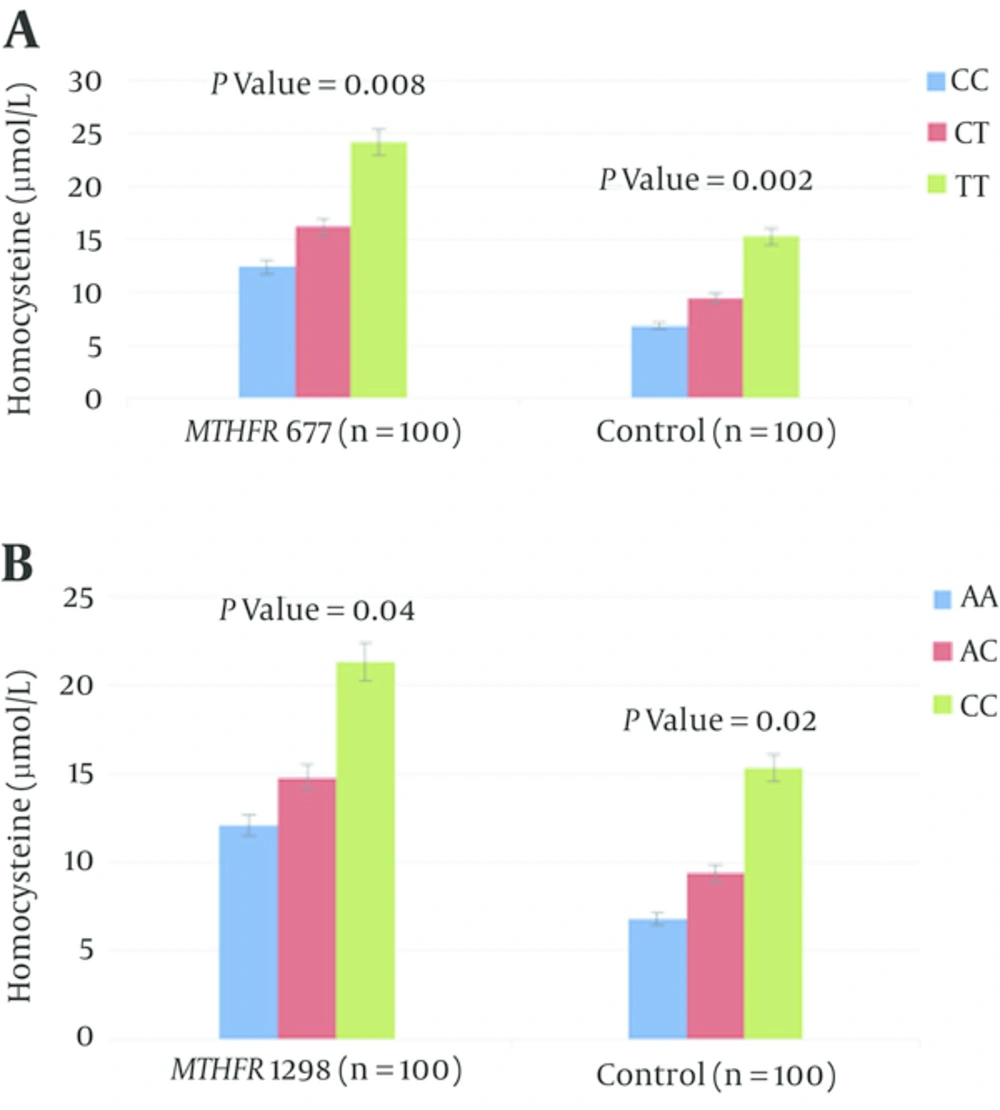
In total, 120 pregnant women at risk of thrombosis and 100 pregnant women without risk of DVT as control group were included in genetic analyses. The age range for pregnant women at risk of DVT between 22 and 38 years and a mean age of 30 years and the age range for pregnant women without risk of DVT between 20 and 35 years and a mean age of 25.2 years. No significant differences were observed in mean age, between the pregnant women at risk of DVT and control subjects (Table 2). Mean plasma homocysteine levels were significantly higher in the pregnant women with DVT (18.3 ± 5.9 μmol/L) than in the pregnant women without DVT (8.9 ± 6.4 μmol/L) (P = 0.021) (Table 2). No significant differences were observed in genotype and allele frequencies, between the pregnant women at risk of DVT and control subjects in C677T and A1298C mutations on the MTHFR gene (Table 3). The allele and genotype frequencies of the C677T and A1298C mutation on the MTHFR gene in DVT pregnant women and controls are given in Table 3. The most frequent MTHFRC677T, and A1298 genotype in pregnant women with DVT was WW with observed frequency of 70.8%, 73.3% respectively, lower frequency was found for WM genotype (15%, 11.6% respectively) and the lowest frequency was found for MM genotype (14.1%, 19.1% respectively). Also, the allele frequency of MTHFRC677T, and A1298C genotype in pregnant women without DVT was WW with observed frequency of 78%, 74 % respectively, lower frequency was found for WM genotype (14%, 10% respectively) and the lowest frequency was found for MM genotype (8%, 16% respectively). T allele frequency (mutant allele in mutation of MTHFRC677T) was 23% and C allele frequency (mutant allele in mutation of MTHFRA1298C) was 32%. The relationship between MTHFR genotypes and plasma concentration of total homocysteine (µmol/L) is significant in both MTHFR 677 and MTHFR 1298 (P = 0.008 and P = 0.04) respectively and controls (P = 0.02) (Figure 2).
| Characteristics | Pregnant Women With DVT (N = 120) | Pregnant Women Without DVT (N = 100) | P Value |
|---|---|---|---|
| 30 (22 - 38) | 25.2 (20 - 35) | 0.251 | |
| 18.3 ± 5.9 | 8.9 ± 6.4 | 0.021 |
aValues are expressed as median (range).
bValues are expressed as mean ± SD.
| Allele or Genotype | Pregnant Women With DVT (N = 120) | Pregnant Women Without DVT (N = 100) | P Value | Odds Ratio | 95% CI |
|---|---|---|---|---|---|
| Wild type | 94 (78.3) | 83 (83) | 0.385 | 0.884 | 0.454 - 1.355 |
| Mutant type | 26 (21.6) | 17 (17) | 0.385 | 1.184 | 0.761 - 1.702 |
| WW | 88 (73.3) | 74 (74) | 0.911 | 0.839 | 0.142 - 5.322 |
| MM | 14 (19.1) | 16 (16) | 0.351 | 1.753 | 0.394 - 7.231 |
| WM | 18 (11.6) | 10 (10) | 0.268 | 0.686 | 0.489 - 1.301 |
| W | 98 (81.6) | 85 (85) | 0.510 | 1.021 | 0.728 - 1.802 |
| M | 22 (18.3) | 15 (15) | 0.510 | 0.871 | 0.548 - 1.352 |
| WW | 85 (70.8) | 78 (78) | 0.227 | 1.437 | 0.832 - 2.312 |
| MM | 17 (14.1) | 8 (8) | 0.151 | 1.638 | 0.858 - 3.023 |
| WM | 18 (15) | 14 (14) | 0.834 | 0.841 | 0.164 - 5.103 |
Abbreviations: M, mutant type; W, wild type.
aValues are expressed as No. (%).
5. Discussion
Our results showed that MTHFRC677T and MTHFRA1289C polymorphisms are not connected with total plasma homocysteine levels in pregnant women with and without DVT. The reasons for this lack of correlation are variable and multi-factorial, such as sample size, number of samples, racial diversity, diet, etc. In cardiovascular disease, increased levels of homocysteine are an independent risk factor that is influenced by factors such as nutritional deficiencies, cancers, drugs and mutations in the MTHFR gene [17-19]. C677T and A1298C mutations on the MTHFR gene are the most fully known mutations that affects homocysteine metabolism [20]. In several papers the polymorphisms C677T and A1298C of MTHFR and fasting plasma homocysteine levels do not seem to be significant risk factors for venous thromboembolic disease [21, 22]. An elevated level of total homocysteine is a risk factor for arterial and venous thrombosis [12, 23-25]. In this study, heterozygous (WM or CT) and homozygous (MM or TT) mutations of MTHFRC677T were not associated with DVT, respectively. In addition, heterozygous (WM or AA) and homozygous (MM or CC) mutations of MTHFRA1298C were not associated with DVT, respectively. T allele frequency in this study (mutant allele in mutation of MTHFRC677T) was 23%, which is higher than south African (10.3%) [26] and Canadian (6%) [27] populations and lower than the frequency reported in Italian (44%) [28] and Turkish (33.6%) [29] populations. Our results were virtually the same results were reported in China Taipei and Brazil 24.4% and 24% respectively [30, 31]. The most frequent MTHFRC677T genotype in pregnant women without DVT was CC (WW) with observed frequency of 70.8% which is higher than those reported by Spiroski et al. lower frequency was found for CT (WM) genotype (15%) which is lower than those reported by Spiroski et al. and the lowest frequency was found for TT (MM) genotype, 14.1% which is higher than those reported by Spiroski et al. [4]. High frequency of MTHFR-677/TT genotype (18% - 19%) was found in several studies conducted in Greece (16.7%) [32], while the lowest frequency (6.2%) was found in Germany [33] and Croatia (6%) [34]. The frequencies of MTHFR-677 CT (WM) and TT (MM) genotypes were slightly increased in pregnant women with deep venous thrombosis, with a consecutive decrease of CC (WW) genotype that the same study was reported by Spiroski et al. [4]. C allele frequency in this study (mutant allele in mutation of MTHFRA1298C) was 33%. The most frequent MTHFRA1298C genotype in pregnant women without DVT was AA (WW) with observed frequency of 74%, which is higher than those reported by Spiroski et al. [4], lower frequency was found for CC (MM) genotype (16%), which is lower than those reported by Spiroski et al. and the lowest frequency was found for CA (WM) genotype, 10%, which is higher than those reported by Spiroski et al. [4]. The frequencies of MTHFR-1298 CA (WM) and CC (MM) genotypes were slightly increased in pregnant women with deep venous thrombosis, with a slightly decreased of AA (WW) genotype that the same study was reported by Spiroski et al. [4]. Non-significant association between MTHFRC677T and MTHFRA1298C alleles, genotypes with deep venous thrombosis found in our study is in agreement with most of the studies [35-41].
In this study we found that plasma total homocysteine level was higher in pregnant women with DVT than in the control group, similar to the previous studies [23, 24, 42, 43].
Oger et al. reported that a mild increase in plasma total homocysteine levels was a risk factor for DVT in abcence of vitamin B12 and folic acid. Slight increase in plasma homocysteine levels was associated with an almost two fold risk of DVT without presence of cancer, surgery and trauma [44]. Meta-analysis of prospective and retrospective studies demonstrates a modest association of homocysteine with venous thrombosis [42]. In several papers the polymorphisms C677T and A1298C of MTHFR and fasting plasma homocysteine levels do not seem to be significant risk factors for venous thromboembolic disease [21, 22]. The results showed that plasma homocysteine levels at baseline were significantly higher for the MTHFR T677T genotype group compared to the MTHFR C677C genotype group. In summary, the association of MTHFRC677T and MTHFRA1289C polymorphisms is connected with total plasma homocysteine levels in pregnant women with and without DVT.