1. Introduction
Exudative pleural effusions include a wide variety of disorders. Many cases are difficult to diagnose. A significant percentage of exudative pleural effusions remain undiagnosed [1]. Various methods are used to identify the cause of unknown’s types. Fluid analysis is the easiest and least invasive diagnostic method. Evaluation of adenosine deaminase activity (ADA) in pleural fluid is inexpensive and easy to do. ADA has been found to be a diagnostic marker in Tuberculous pleural effusion (TB-PLE) since1978 [2]. However pleural ADA levels are also in different type of lymphocytic exudative pleural effusions [3]. A lot of studies have focused on the applications of ADA values in diagnosis of pleural tuberculosis [4]. Test efficacy of pleural ADA in diagnosis of TB-PLE is reported in wide range from 0 to 100% [3]. Some researchers believe that the pleural ADA levels of more than 70 IU/L required for the diagnosis of TB-PLE, while a level less than 40 IU/L actually ruled out TB-PLE [3]. Versus of this idea is suggestion a pleural fluid ADA value < 16.81 IU/L for excluding TB-PLE [5]. Several factors may affect the pleural fluid ADA levels in patients with TB-PLE. Among these factors are prevalence of tuberculosis, race of studied population, age and immune status of the patients [6]. Pleural fluid cyto-analysis, pleural protein and LDH may also affect the ADA levels in patients with TB-PLE [3, 7]. Due to variability of pleural fluid ADA measures in TB-PLE, researchers have recommended the necessity of regional studies for determination of specific regional values [5, 7]. The aim of the present study was to evaluate the value of pleural ADA in diagnosing of TB-PLE in South Khorasan province. The findings will help physician in interpretation of pleural ADA in differentiation of TB-PLE from other lymphocytic exudative pleural effusion.
2. Methods
A cross-sectional descriptive-analytic study was designed. The study was conducted in Vali-e-Asre hospital in South Khorasan province (east of Iran) started since 2008 for a period of 6years. Vali-e-Asre hospital, serves as the only tertiary referral center in South Khorasan province. The study population was adults > 12 years old with significant pleural effusion on chest x ray (CXR) study, who needed diagnostic measures. The informed consent was obtained in all patients for invasive diagnostic procedures. Pleural tap was undertaken and 30 mL of fluid was evacuated in the heparin-treated syringe and transferred to laboratory for analysis. Exudative pleural effusion was labeled according to the criteria of light (either effusion protein/serum protein ratio greater than 0.5 or effusion lactate dehydrogenase (LDH)/serum LDH ratio greater than 0.6) [8].
All patients enrolled in the study were those with exudative pleural effusion and all cases with transudative pleural effusion were excluded from the study. Extensive clinical, radiological, biochemical, and pathological investigations were performed to determine the cause of the effusion. Diagnosis of TB-PLE was considered in differential diagnosis of all lymphocytic exudative fluids and confirmed either by histopathology of plural biopsy (Granulomatous reactions) or Ziehl-Neelsen staining of pleural fluid sediment. TB-PLE was also labeled by Ziehl-Neelsen staining of sputum and bronchial washingin patients who had chronic infiltration on CXR in concomitant with lymphocytic exudative pleural effusionin a situation wherethe meticulous investigations did not suggest any alternative diagnosis. Appropriate treatment was also started for each treatable patient and all patients were followed. Response to anti-TB was used as another confirmation for correct diagnosis of tuberculosis.
Total ADA was determined calorimetrically by Diazyme’s ADA assay [9].
All collected data were recorded on a special sheet. SPSS version 16 software was used for data analysis. Mean differences between groups were analyzed using student t test and One-Way ANOVA (Tukey test).The χ2 test was used to determine difference in frequencies between groups.Pearson correlation coefficient was used for defining correlation between ADA and independent variable. ROC curve and Youden index was used to define usefulness and optimal cut off point of the ADA levels in diagnosis of tuberculosis pleural effusion.
Correlation coefficient (r) defined as negligible (< 0.3), weak (0.3 - 0.5), moderate (0.5 - 0.7), high (0.7 - 0.9), very high (> 0.9). A P value of < 0.05 was considered to be statistically significant.
3. Results
A total of 255 cases are enrolled during study period. There were 139 (54.5%) males and 116 (45.5%) females, with a mean age of 63.8 ± 18 years (range 17 to 91 years). Gender distribution of various exudative pleural effusions in studied patients is showed in Table 1.
| Cause of Exudative Pleural Effusion | No. (%) | Male | Female |
|---|---|---|---|
| Malignant pleural effusion | 106 (41.6) | 52 (49.1) | 54 (50.9) |
| Para pneumonic and empyema | 29 (11.4) | 19 (65.5) | 10 (34.5) |
| Tuberculosis pleural effusion | 36 (14.1) | 19 (52.8) | 17 (47.2) |
| Vasculitis | 9 (3.5) | 6 (66.7) | 3 (33.3) |
| Pulmonary embolism | 10 (3.9) | 7 (70) | 3 (30) |
| lymphoma | 11 (4.3) | 7 (63.6) | 4 (36.4) |
| Hydatid cyst | 2 (0.8) | 2 (100) | 0 |
| Othersa | 52 (20.4) | 27 (51.9) | 25 (48.1) |
Gender Distribution in Different Etiologies of Pleural Effusion
The mean ADA level in studied patients was 25.2 ± 33.3 IU/L. The patients divided to 36 (14.1%) cases with TB-PLE and 219 (85.9%) cases with non-TB-PLE. The mean age in TB and non-TB-PLE was 63.1 ± 19.1 and 63.9 ± 17.9 respectively (PV = 0.87). Biochemical parameter (LDHU/Land Proteing/dL), ADA levels and cell count in various causes of Pleural effusion of studied patients were presented in Table 2. The mean ADA levels in TB and non-TB-PLE was 40.2 ± 24.7 and 22.7 ± 33.9 IU/L respectively (PV = 0.003) (Table 2).
| Pleural Fluid Parameter | TB | Non-TB | P Value |
|---|---|---|---|
| ADA, IU/L | 40.2 ± 24.7 | 22.7 ± 33.9 | 0.003 |
| Sugar, mg/dL | 80.5 ± 41.6 | 105.2 ± 58 | 0.018 |
| Protein, g/dL | 4.7 ± 0.086 | 3.8 ± 1.37 | 0.00 |
| LDH, U/L | 1235 ± 1387 | 862.2 ± 1430 | 0.14 |
| Cell count, cell/mL | 2271.5 ± 2871.2 | 2388.8 ± 6259.1 | 0.91 |
Biochemical Parameter, ADA Levels and Cell Count (WBC) in Pleural Fluid (TB-PLE vs non TB-PLE)
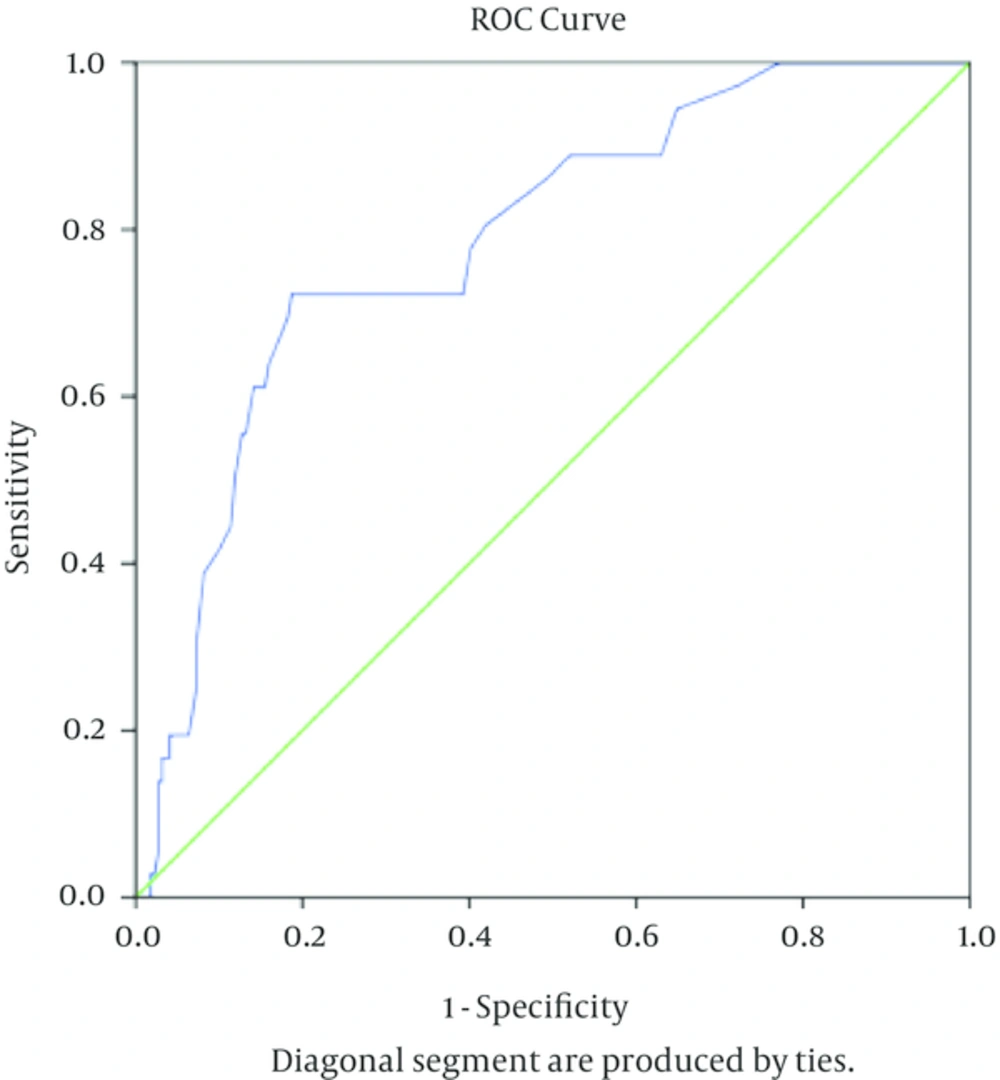
Considering the cut-off point of 35 IU/L for ADA in the diagnosis of TB-PLE (cut-off point value used routinely in clinical practice), sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and Youden index were calculated and showed in Table 3. Using ROC Curve and Youden index, the area under the ROC for ADA in diagnosis of TB-PLE was 0.786 (Standard error = 0.039, CI = 0.71 - 0.86, P < 0.001) and optimal cut-off point of pleural ADA for diagnosis of TB-PLE was 30 IU/L (Figure 1).
The area under the ROC for ADA in diagnosis of TB-PLE was 0.786 and optimal cut off point of pleural ADA for diagnosis of TB-PLE was 30 IU/L. At this value of ADA, the sensitivity is 0.786 and specificity is 0.812 (1 - specificity = 0.188).
At this cut-off point (optimal cut-off point for pleural ADA in TB-PLE diagnosis in the present study), the value of pleural ADA had a sensitivity of 72.2%, and specificity of 81.2% for the TB-PLE diagnosis (Table 3).
| Base of Test Effectiveness | Cut-Off Point of 30 IU/L (95%CI) | Cut-Off Point-of 35 IU/L (95%CI) |
|---|---|---|
| sensitivity | 72.2 (CI = 0.54 - 0.85) | 61 (0.43 - 0.76) |
| specificity | 81.2 (CI = 0.75 - 0.86) | 86 (CI = 0.80 - 0.90) |
| positive predictive value | 39 (CI = 0.27 - 0.51) | 42 (CI = 0.28 - 0.55) |
| negative predictive value | 95 (CI = 0.90 - 0.97) | 93 (CI = 088 - 096) |
| Youden index | 0.53 | 0.47 |
Sensitivity, Specificity, PPV and NPV Value of Pleural ADA in Tuberculous Pleural Effusion
Pleural fluid ADA levels in TB-PLE revealed a negative correlation with age (r = -0.40, P = 0.01), but positive correlation with pleural fluid protein (r = 0.44, P = 0.007) and LDH (r = 0.32, P = 0.05). This situations with slight difference was also observed in neutrophilic exudative pleural effusion and also when all patients including tuberculosis and non-tuberculosis patients were placed altogether in one group. The lowest and highest ADA value in TB-PLE group was 9 IU/L and 124 IU/L in 81 and 25 years old woman respectively. The mean ADA levels in age groups < 45 and > 45 of patients with TB-PLE were 55.6 ± 30 IU/L and 35.1±20.8IU/L respectively (P = 0.02). The mean levels of ADA in massive pleural effusion of patients with TB was 40.6 ± 29.7 IU/L versus 39.5 ± 20.7 IU/L in compared with non-massive pleural effusion (P = 0.9).
The causes of exudative pleural effusion among non-tuberculosis studied patients included 106 cases (41.6%) with malignant and 29 cases (11.4%) with acute bacterial infection. The mean age and mean ADA levels in each etiological group are described in Table 4.
| Ethiology of Exudative | No. (%) | Agea | Statistically Significant at (α) Level < 5% | ADA (Means)a | Statistically Significant at (α) Level < 5% |
|---|---|---|---|---|---|
| Malignant | 106 (41.6) | 66.6 ± 14.6 | 1 with 6 | 17.9 ± 12.3 | 1with 6 and 3 |
| Parapneumonic and empyema | 29 (11.4) | 60.9 ± 22.2 | Not significant difference | 30.6 ± 35.5 | Not significant difference |
| Tuberculosis | 36 (14.1) | 63.1 ± 19.2 | Not significant difference | 40.3 ± 24.7 | 3 with 1 |
| Vasculitis | 9 (3.5) | 57.2 ± 13.5 | Not significant difference | 17.6 ± 11.8 | 4 with 6 |
| Pulmonary embolism | 10 (3.9) | 64.8 ± 13.9 | Not significant difference | 12.3 ± 9 | 5 with 6 |
| Lymphoma | 11 (4.3) | 44.8 ± 25.5 | 6 with 1 and 8 | 63.8 ± 116.2 | 6 with 1 and 8 and 4 and 5 |
| Hydatide Cyst | 2 (0.8) | 52 ± 43.8 | Not significant difference | 71.5 ± 92.6 | Not significant difference |
| Othersb | 52 (20.4) | 65.8 ± 17.9 | 8 with 6 | 20.5 ± 22.5 | 8 with 6 |
Mean of ADA and Age of Studied Patients
4. Discussion
The present study revealed that mean value of ADA in TB-PLE, although not the highest, but was significantly higher than the most prevalent, mainly malignant pleural effusion. Assessment of ADA levels in pleural effusion is a simple and inexpensive test which has been introduced for diagnosis of TB-PLE [2]. However various studies presented different values in TB-PLE. Some researchers have concluded that the difference in ADA levels is due to difference in TB prevalence [6]. A study conducted in the neighboring of our region, Zahedan, has shown mean ADA levels of 39.63 ± 14.96 IU/L and 22.11 ± 9.33 IU/L in TB-PLE and non-TB-PLE respectively [10]. In this respect the results of the study conducted in Zahedan are in consistent with our study. They also considered cut-off value of 35 IU/L for TB-PLE diagnosis and showed sensitivity and specificity of 70.3% and 91.3% respectively versus our sensitivity and specificity of 61% and 86%. Given that in areas with high prevalence of one disease, the more sensitive test is more important, the cut off value of 35 IU/L for ADA in Zahedan will be more valuable. In contrast, a study conducted in Tehran showed much higher levels of pleural ADA in TB-PLE than our and also the patients from Zahedan. The total ADA activities in patients with TB-PLE was 46 U/L and more in that study [11]. Also, in another study from Tehran, ADA level in TB-PLE was higher than our study [12]. Similarly, the studies conducted in different area of the world, have shown that the values of ADA in TB-PLE are higher than values of patients studied in our region and also in Zahedanm [6, 10]. The south Khorasan and Zahedan in Iran, are among regions with relatively high prevalence of tuberculosis [13]. Despite some similarity in prevalence of TB among our region and Indian population, the mean pleural fluid ADA levels of our patients is reported to be lower than those from Indian population [6, 13-15]. Pleural fluid ADA levels in our patients were also reported lower than those from regions with low prevalence of tuberculosis such as Japanese and Albania and regions with intermediate prevalence of TB such as Portugal and Argentina [6, 16-19]. On the other hand in Thailand (an Asian country with high prevalence of TB), the pleural fluid ADA level in patients with TB-PLE is higher than the levels reported in our study [20]. Most researchers believe that ADA could be an excellent tool in TB-PLE diagnosis in areas with high prevalence of tuberculosis [21]. However this is not enough to explain the discrepancies in the results of ADA measurements in different studies. A study which consider different prevalence scenarios for TB, revealed no difference in pleural ADA levels between tree study periods with different TB prevalence and claim that ADA can be useful for the diagnosis of TB-PLE even in area with low-to-intermediate prevalence of TB in patients with lymphocytic exudative pleural fluid [22].
Another factor that affects the ADA levels in TB-PLE was age of the patients in our study. The mean age of patients with TB-PLE in our study was high. Correlation coefficient analysis showed a significant but weak and negative correlation between pleural fluid ADA level and age in all and also in patients with TB-PLE in particular. In comparison to other studies conducted in Europeans, Indian, and Japanese patients, and even in comparison to study conducted in Tehran, the patients from our region seems to have much lower levels of ADA in TB-PLE [16, 23].
It may be explained by the subject that the mean age of patients with TB-PLE was high in our study. This finding has been also approved by other studies conducted by some researches [6, 24-26]. Our patients had also older age than those from Zahedan [10]. It could be also an explanation for relatively lower values of ADA in our TB-PLE than those patients from Zahedan.
There was significant, but weakly positive correlation between Pleural fluid ADA activity, protein and LDH in TB-PLE and also non-TB-PLE exudative pleural effusion altogether. Regardless of whether pleural effusions are due to TB or non-TB-PLE, the pleural fluid LDH and protein are markers of the inflammatory reactions in the pleural fluid. The positive correlation between these factors and ADA would be explained according the proposition offered by Lee et al. [27]. It is assumed that ADA activity in pleural fluid is further related to antigen stimulation and cell proliferation than to amount of lymphocytes present. Our results are somewhat inconsistent with the results observed by Kashiwabara et al. [28]. While Kashiwabara et al demonstrate positive and significant correlation between ADA and LDH; they could not show correlation between ADA, protein and age in patient with exudative pleural effusion.
We are faced with the greatest amount of ADA activity in lymphoma-related pleural effusion. Tuberculosis and Lymphoma are two important causes of lymphocytic effusions. It is often challenging to differentiate the two conditions. ADA activity is mainly dependent on the lymphocyte stimulation and proliferation. As in TB-related pleural effusion, the results about ADA activity in lymphoma-related pleural effusion are also heterogeneous. While some researchers believe that there is overlap between tuberculosis and lymphoma [29] others have concluded lower levels of ADA activity in lymphoma [30, 31]. On the other hand, Porcel JM et al suggest that an extremely high ADA activity should raise suspicion of empyema or lymphoma [21]. Lymphoma- related pleural effusion in our study had a younger age than those with pleural tuberculosis. This could explain higher ADA activity in lymphoma-related pleural effusion in our study.
We had two cases of hydatid cyst ruptured into the pleural space, one in 21 years old man complicated by bacterial infection and one in 81 years old man without such complication. The highest value of ADA activity in pleural fluid was observed in case complicated by bacterial infection. Young age and Concurrent superimposed bacterial infection maybeinducedthe highest levels of pleural fluid ADA activity in such special case.
One limitation of the present study was the low number of tuberculosis patients so that despite of significant negative correlations observed between pleural ADA and age, but correlations between pleural ADA and inflammatory markers (pleural protein and pleural LDH) were weak in the present study, and a definitive conclusion of correlations could not be synthesized now.
4.1. Conclusions
Mean and optimal cut-off point of pleural ADA in TB-PLE in our study was among the lowest value in around the world. We emphasize on the elderly people in our study. In addition, pleural protein and LDH should be also considered in interpreting of pleural ADA levels. It is recommended to carry out studies with larger number of patients to make a more definitive interpretation of influence of inflammatory markers on the pleural ADA levels.