1. Background
Resistance of pathogenic bacteria to antibiotics is a problem that has attracted interest all over the world. This resistance to various antimicrobial agents occurs through inherent and acquired resistance mechanisms. Acquired resistance results from contact with antimicrobial agents [1]. Utilization of the antibacterial property of plants has offered a new approach for coping with resistance to antibiotics and for their replacement. Nowadays, four billion people the world over use plants as a source of pharmaceutical materials, and 25 percent of the standard drugs prescribed by physicians are of plant origin. Plants have unlimited capability in synthesizing various aromatic compounds, phenolic compounds and their derivatives [2, 3]. Therefore, it is necessary to study the active compounds in plants of all geographical regions. Rumex alveolatus grows in the mountainous regions of western Iran at altitudes of 1200 - 1400 meters. It has long, straight roots, fleshy leaves, and green flowers that are carried above the leaves in clusters. In traditional medicine, this genus is used to treat tumors, hepatic diseases, constipation, heart problems, spleen diseases, hiccup, flatulence, asthma, bronchitis, indigestion, toothache, gall, and nausea [4, 5]. Rumex contains many bioactive materials such as flavonoids and anthrakinones and, especially in its roots, carotenoids, vitamins (particularly vitamin C), proteins, lipids, and organic acids have been identified [6, 7]. Antimicrobial properties of aqueous and methanol extracts of various plant species against a broad spectrum of bacteria have been reported from various geographical regions. We studied antibacterial activity of R. alveolatus before. We reported methanol extract of R. alveolatus leaves were effective against Pseudomonas aeruginosa at 31.3 mg/mL [8]. In another study reported that the minimum inhibitory concentrations (MIC) of ethanol extract of R. alveolatus against Escherichia coli and Pseudomonas aeruginosa were 50 and 25 mg/mL [9]. Results of research by Nisa demonstrated the butanol extract of R. dentatus had antibacterial effect against E. coli, P. aeruginosa, and S. aureus. Research on extract of Rumex attributed its antibacterial properties to the presence of compounds including flavonoids, glycosides, reducing sugars, anthrakinones, tannin, and alkaloids [10]. Therefore, this research was conducted to identify active compounds and antibacterial activity and anti-quorum sensing effects of aqueous and methanol extracts of R. alveolatus leaves and roots against a number of bacteria.
2. Methods
This empirical study was carried out from April 2015 to October. The fresh plant, R. alveolatus was collected Lorestan Province (47°36’, 33°32). This plant was identified by plant taxonomy experts (registration number 025/006/001). The dried plants were powdered and kept in dark-colored containers in a refrigerator at 4°C.
The 60 grams of powder were extracted with 300 mL of methanol using Soxhlet apparatus for eight hours. Then a rotary evaporator was used to dry the powder at 40°C. The concentrated extracts were diluted to 500, 250, 125, 62.5, 31.3 and 15.6 mg/mL by 5% dimethyl sulfoxide (DMSO, Merck) and were used in antibacterial experiments.
The strains of S. aureus (ATCC:25923), E. coli (ATTC:25922), K. pneumoniae (ATCC:10031), S. typhi (ATCC:1690), S. sonnei (ATCC:1188), P. aeruginosa (ATCC:1310), and A. baumannii (ATCC:10654) obtained from the Collection of Microorganisms at the Iranian research organization for Science and technology. The bacterial suspension turbidity was adjusted to be identical to that of 0.5 McFarland turbidity standards (equivalent to 1.5 × 108 CFU/mL). To make sure of the bacterial concentration, absorption was adjusted in a spectrophotometer (UNIC-UV-2100, USA) at 630 nm in the 0.08 - 0.1 range. Following that, 0.2 mL of the suspension was added to 19.8 mL of MHB so that the bacterial cell concentration reached 1.5 × 106 CFU/mL [11].
2.1. Antibacterial Activity
The well diffusion method was used to determine the antibacterial activity of the extract. One hundred µl of the bacterial suspension (1.5 × 106 cfu/mL) were spread on MHA medium, and then wells were made. Each well was inoculated with 100 µL of each prepared concentration of the extract. The negative control was the 5% solution of DMSO, and the positive control was imipenem solution (10 µg/mL). All of the plates were incubated at 37°C for 24 hours. The MIC and MBC of the extracts were determined using the microtiter plate method [12, 13].
2.2. Anti-Quorum Sensing Activity of Methanol Extract of R. alveolatus
The anti-quorum sensing assay was performed in sub-MIC concentrations of the methanol extract of R. alveolatus with no interference on bacterial growth.
2.3. Anti-Biofilm Activity
The methanol extract were first prepared at 62.5, 31.3 and 15.6 mg/mL in MHB that contained 1% glucose. One hundred µL of each extract concentration were poured into wells in columns 1 to 8 of the sterile flat bottom of 96 well. Then 150 µL of the bacterial suspension (1.5 × 108 CFU/mL) were added to each well, and the 100 µL of the culture medium containing 1% glucose were also transferred to each one. The 250 µL of sterile MHB were poured in column 11 as the negative control, and 150 µL of the bacterial suspension and 100 µL of the MHB containing 1% glucose to column number 12 as the positive control. After inoculation, the absorbance of the samples was read using ELISA tray reader at 492 nm. The microplates were placed in an incubator at 37°C for 72 hours, and then the contents in the wells were discarded. Following that, 250 µL of 95% ethanol were poured into each well to fix the cells. After 15 minutes, the ethanol in the wells was removed, and the wells were dried in the ambient air. The 200 µL of 2% crystal violet dye were added. After five minutes, the extra dye was then washed off under a gentle stream. The microplates were dried at room temperature, and then 100 µL of 33% glacial acetic acid were poured into each well. The microplates were put in an incubator at 37°C for 20 minutes. The absorbance by the wells was read at 492 nm. Reduction of biofilm formation was calculated [14, 15].
2.4. Pyocyanin Production Test
The effects of the methanol extract of R. alveolatus on pyocyanin production of P. aeruginosa were determined as Krishnan et al. [16]. The 250 µL of methanol extract of R. alveolatus at concentrations of 62.5, 31.3 and 15.6 mg/mL were prepared and 100 µL of the bacterial suspension (1.5 × 108 CFU/mL) was added. The test tube containing the bacterial suspension without the extract was considered as control. The tubes were incubated at 37°C for 24 hours, were then centrifuged at 8000 rpm at 4°C, and the supernatant was transferred to another sterile microtube. One mL of chloroform was added to 5 mL of the supernatant, and the supernatant was discarded. The absorbance of lower part of the microtubes was read at 690 nm. The reduction of pyocyanin production was calculated.
2.5. Protease Activity Test
The effect of the methanol extract of R. alveolatus on protease activity of P. aeruginosa was evaluated by Laurie method [17]. The 250 µL of the methanol extract of R. alveolatus at concentrations of 62.5, 31.3 and 15.6 mg/mL were prepared and 100 µL of the bacterial suspension (1.5 × 108 CFU/mL) were added. The tube containing the bacterial suspension without the extract was the control. The test tubes were incubated at 37°C for 24 hours and centrifuged at 8000 rpm at 4°C for 10 minutes. Then 100 µL of the supernatant were added to 900 µL of 0.5% azocasein. After 30 minutes at 37°C, 250 µL of 15% trichloroacetic acid were added to the tubes. The tubes were centrifuged at 8000 rpm for 10 minutes at 4°C. The absorbance of supernatant was read at 440 nm. The reduction in protease activity was calculated.
2.6. Gas Chromatography/Mass Spectrometry (GC/MS) Analysis
An Agilent model 7890 GC interfaced to a 5975C mass selective detector was used for mass spectral identification of the elements of the extract.
2.7. Statistical Analysis
Data were analyzed using the statistical package for social sciences (SPSS-Ver.17). Normality of the data was evaluated by the Shapiro-Wilk test. Non-parametric kruskal wallis test was used to compare the values between the groups due to the lack of normal assumption. A P value less than 0.05 were considered significant.
3. Results
Sensitivity of the strains to methanol extracts of R. alveolatus leaves and roots was studied using the well diffusion method. Results were presented in Table 1. The root methanol extract of R. alveolatus did not exhibit any antibacterial effects, except against S. aureus.
| Sample | Concentrations of the Methanol Extract, mg/mL | Negative Control | Positive Control (Imipenem) | |||||
|---|---|---|---|---|---|---|---|---|
| Leaves | Roots | |||||||
| 500 | 250 | 125 | 500 | 250 | 125 | |||
| P.aeruginosa | 22.8 ± 0.6 | 21.4 ± 0.6 | 19.3 ± 1.5 | - | - | - | - | 26.0 ± 1.4 |
| S.typhi | 12.1 ± 1.0 | 9.3 ± 0.6 | 6.66 ± 0.6 | - | - | - | - | 30.0 ± 2.6 |
| A.baumannii | - | - | - | - | - | - | - | 27.0 ± 2.1 |
| S.sonnei | - | - | - | - | - | - | - | 28.0 ± 1.8 |
| K.pneumoniae | - | - | - | - | - | - | - | 26.0 ± 2.4 |
| E. coli | - | - | - | - | - | - | 26.0 ± 1.9 | |
| S. aureus | 10.7 ± 1.3 | 9.7 ± 1.5 | 8.7 ± 1.5 | 22.0 ± 1.0 | 17.6 ± 0.6 | 11.7 ± 1.5 | - | 25.6 ± 2.1 |
The species of bacteria and extract concentration (P < 0.05) influenced zone of inhibition. Furthermore, increases in the concentration of the methanol extracts of leaves and roots improved their antibacterial activities. Among the studied concentrations of these extracts, zone of inhibition at 500 mg/mL were significantly larger compared to the other concentrations (P < 0.05). Based on Duncan post hoc test, all concentrations were compared in pairs and with negative and positive controls. In P. aeruginosa, S. aureus, and S. typhi, the various concentrations were significantly different from the negative control (P < 0.05), in all the studied bacterial strains significant differences were observed between zone of inhibition at 500 and 125 mg/mL concentrations of the methanol extracts of leaves and imipenem (P < 0.05). The methanol extracts of R. alveolatus leaves did not affect E. coli, K. pneumoniae, S. sonnei and A. baumannii.
In S. aureus, inhibition zone diameters created by methanol extract of R. alveolatus roots at 125, 250, and 500 mg/mL were significantly different, and all the paired comparisons between the concentrations were significant. Inhibition zone diameters of S. aureus produced by root extract were significantly larger compared to leaf extract (P < 0.05).
The MIC of the methanol leaves extract was 125 mg/mL for P. aeruginosa, S. aureus, and S. typhi, and the MBC was 250 mg/mL. The MIC of the root extract for S. aureus was 125 mg/mL and the MBC was 250 mg/mL. the aqueous extracts of R. alveolatus leaves and roots did not exhibit any antibacterial effects.
3.1. Anti-Quorum Sensing Activity of Methanol Extract of R. alveolatus
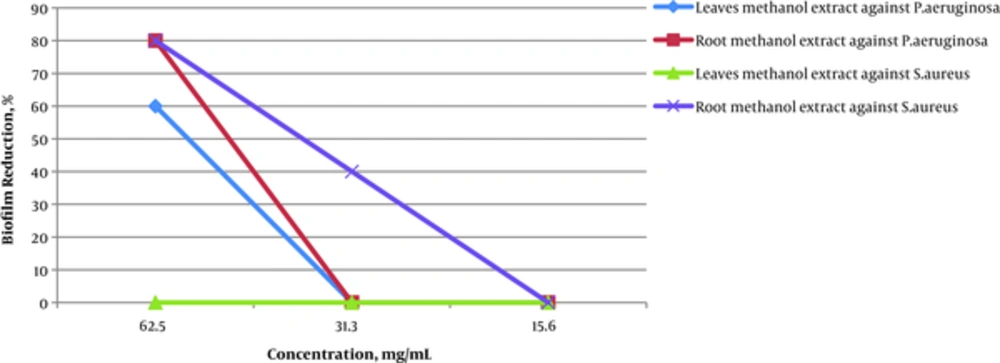
Results confirmed that leaves extract of R. alveolatus were capable of reducing biofilm formation by P. aeruginosa and S. aureus at sub-MIC of methanol extract. The methanol extract of R. alveolatus leaves and roots, at 62.5 mg/mL reduced biofilm production in P. aeruginosa by 60 and 80 percent, respectively. However, at 62.5 mg/mL, methanol root extract of R. alveolatus lowered biofilm production in S. aureus by 80 percent; and, at 32.3 mg/mL, the methanol extract of R. alveolatus leaves decreased biofilm production in S. aureus by 40 percent.
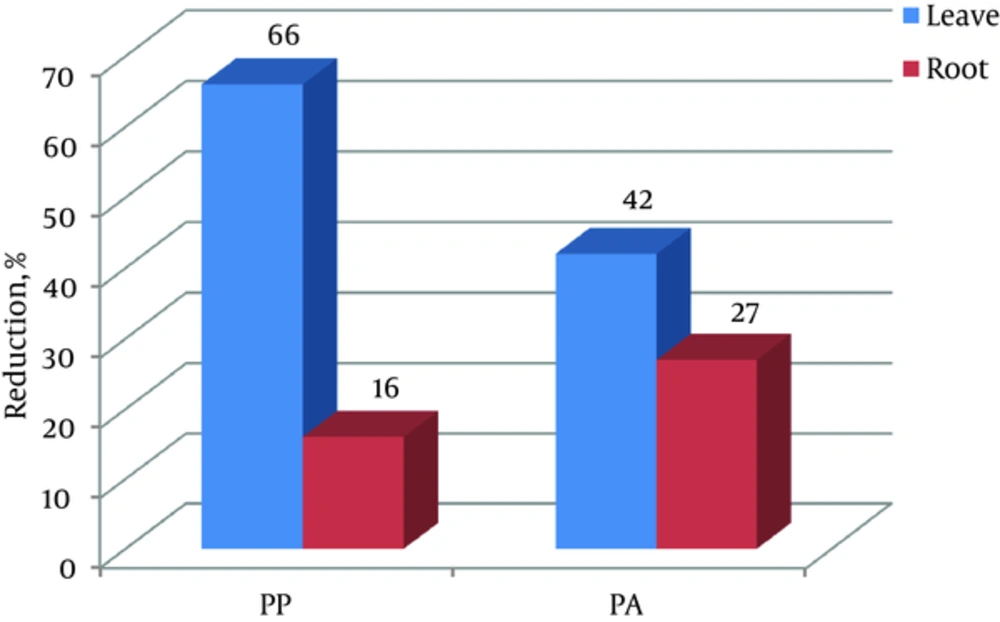
As shown in Figure 2, methanol extract of R. alveolatus leaves at 62.5 mg/mL reduced pyocyanin production by 66 %, while methanol extract of the roots of this plant at 62.5 mg/mL decreased pyocyanin production by up to 16 percent. Methanol extracts of R. alveolatus leaves and roots at 62.5 mg/mL reduced protease activity by up to 42% and 27%, respectively. The lower concentration of methanol extract did not show any effect on pyocyanin production and protease activity.
The methanol extract of R. alveolatus taken from GC / MS spectrum were analyzed. The components of methanol extract were identified in Table 2. The percentage of each product in the extract was shown in this table indicating Biocyclo (3.1.1) heptan-3- one, 2, 6, 6- trimethyl (1.alpha, 2alpha, 5a) with 97% had the most presence in our extract. Table 2 also reveals high probability of Alpha-Pinene, Sabinene and Eucalyptol (1, 8-cineole) that proved antimicrobial activity in the extract.
| Identified Compounds | Percentage of Total | Kovats Retention Index | Release Time |
|---|---|---|---|
| 5-Methyl-2-cyclohexanone | 0.42 | 1156.03 | 16.765 |
| 4-Methyl-1-cyclohexane | 0.28 | 1393.53 | 20.539 |
| Alpha-pinene | 0.21 | 929.94 | 7.988 |
| 5-Methyl-2-cyclohexanol | 0.75 | 1275.08 | 17.409 |
| Cyclononasiloxane-ctadecamethylcy | 0.22 | 1826.72 | 30.009 |
| 1,2-benzenedicarboxylic acid | 89.68 | 2519.18 | 34.264 |
| Toluene | 3.27 | 780. 25 | 4.147 |
| 1-Methyl-3 benzene | 0.1 | 1024.52 | 11.425 |
| Eucalyptol | 0.09 | 1030.20 | 11.693 |
| Caryophyllene | 0.11 | 1420.33 | 23.198 |
| ethylhexyl phthalate | 0.27 | 2516.27 | 34.176 |
| 2,6-dimethyl cyclohexanol | 0.20 | 1108.47 | 15.262 |
| 2-methyl-5H-dibenz | 0.70 | 2747.41 | 35.116 |
4. Discussion
In this research, a qualitative method (well diffusion) and a quantitative method (microdilution) showed antibacterial and anti-querumsensing of R. alveolatus. Results indicated that zone of inhibition had a direct relationship with extract concentration in the well diffusion method (P < 0.05). Furthermore, means of inhibition zone diameters for the various bacterial species were significantly different in this method (P < 0.05). In other words, bacterial species influenced zone of inhibition.
P. aeruginosa was the most sensitive species to methanol extracts of R. alveolatus leaves, while S. aureus exhibited the maximum sensitivity to the methanol extract of R. alveolatus roots. This difference in sensitivity of the various bacteria to the antimicrobial compounds was probably due to differences in the cell wall of the microorganisms. McKeegan et al. showed that Gram-negative bacteria were more resistant to extracts compared to Gram-positive bacteria.
Our results conform to those previous research, who reported that methanol extracts of R. alveolatus leaves at 250 mg/mL created inhibition zone diameters of 12.5 and 20.67 mm for S. aureus and P. aeruginosa, respectively [18]. At 250 mg/mL, the methanol extract of this plant in the present research created inhibition zone diameters of 9.68 and 21.4 mm against S. aureus and P. aeruginosa, respectively. These results indicate that the effective compounds in methanol extracts of R. alveolatus leaves have very good antimicrobial effects against P. aeruginosa, while they do not exhibit suitable antibacterial activity against S. aureus. It seems that the active compounds present in the methanol extract of R. alveolatus leaves have different mechanisms of action against Gram-negative and Gram-positive bacteria.
In research conducted by Moradi, ethanol extract of R. alveolatus at 300 mg/mL created inhibition zone diameters of 25.33 and 16.37 mm against P. aeruginosa (ATCC: 85327) and S. aureus (ACTT: 25923), respectively. However, results of the present study do not confirm this for other Gram-negative bacteria. The methanol extract of R. alveolatus leaves had no antimicrobial effects against Gram-negative bacilli of E. coli, K. pneumoniae, S. sonnei, and A. baumannii, and only created an inhibition zone diameter of 12.1 mm against S. typhi. Almost all known antimicrobial compounds of plant origin are either aromatic or organic substances, and most of them are extracted using methanol and ethanol solvents. The researchers have shown that extracts prepared by using organic solvents have greater and more stable antimicrobial effects because most known active antimicrobial compounds are insoluble in water and, therefore, extracts prepared by using organic solvents are more capable in extracting antimicrobial substances [18]. Researchers have attributed antibacterial properties of R. alveolatus to its phenolic compounds. This is probably why aqueous extracts lack antibacterial effects because they have concentrations of phenolic compounds dissolved in water. This is in agreement with studies conducted by Nisa and Yildirim [5, 19]. The differences in the mentioned results are due to the different plant species used in the first place and to differences in the tested parts of the plants, in the tested bacterial strains and/or in the habitats of the plants in the second place. Presence of phenolic compounds in plant extracts is one of the main reasons for their antimicrobial effects [20].
In the present research, 1, 2-benzenedicarboxylic acid constituted 89.68 percent of the main compounds isolated from R. alveolatus using the GC-MS method (Table 2). The large content of phenolic compounds in our research is related to the antibacterial properties of this extract. Studies have indicated that polyphenols including tannins, anthrakinones, and flavonoids have effective antimicrobial effects against various microorganisms in addition to their antioxidant properties [21, 22]. Therefore, considering the presence of anthraquinones and flavonoids in plants of the Polygonaceae family, antimicrobial effects of methanol extracts of R. alveolatus leaves and roots can be attributed to these compounds [23-27].
The efficacy of the methanol extract in reducing biofilm formation by the mentioned bacteria was confirmed. It is observed that presence of endol together with terpenes in extracts enhanced their bactericidal properties [28]. Considering the large content of phenolic compounds in R. alveolatus, its inhibition of biofilm formation seems to be logical.
In our research, extracts of R. alveolatus reduced activities of protease and decreased pyocyanin production in P. aeruginosa. Pathogenicity of bacteria is strongly associated with their ability in secreting various toxic and decomposing enzymes into the related environment. Among these enzymes, lipase and protease become involved in bacterial pathogenicity through breaking down host defenses and non-specific physical barriers. As extracellular enzymes in P. aeruginosa, proteases provide the necessary nutrients for bacterial growth.