1. Background
Multiple sclerosis (MS) is an autoimmune disease of the central nervous system which leads to severe neurological disability due to axonal loss. It is more common in young adults. Women are affected nearly twice as often as men [1]. Several clinical courses are described in MS including relapsing-remitting (RRMS), secondary-progressive (SPMS), primary-progressive and progressive-relapsing (PRMS) [2].
The pathogenesis of MS is not well understood, but many epidemiological studies have compared incidence and prevalence rates of the disease across different areas of the world that suggest a complex environment–genotype interaction in etiology of MS [3]. The increased recurrence risk within families indicates roles for genetic factors in the etiology and susceptibility to the development and course of MS [4].
Association with the human leucocyte antigen (HLA) genes from the major histocompatibility complex on chromosome 6p21.3 was identified almost 45 years ago. In particular, the HLA-II gene DRB1*1501 has consistently been found associated with MS [5].
Although several studies have been carried out on the association of DRB1*1501 with MS in Iran [6-9], there are no data so far about this issue in Khuzestan province. Given the ethnicity diversion in Khuzestan province and also due to the fact that unlike Persians, Arabs in Iran are mostly located in Khuzestan and since there are a few data in this regard in Arabic countries, this population study can be of great importance. From this point of view, this article aimed to use genomic typing techniques to evaluate association between HLA-DRB1*1501 and a cohort of MS patients who were resident locating southwest of Iran. In addition, this study examined the probable relationship between the allele with course of disease, ethnicity, expanded disability scale score (EDSS) [10] and gender.
2. Methods
Two hundred patients participated in this case-control study. Peripheral blood was collected from the patients in Khuzestan province who had registered in the Khuzestan MS community according to the McDonald criteria that was used for diagnosis of MS. Detailed clinical and laboratory information was provided from the participants who were enrolled in the present study after obtaining informed consent. A questionnaire was supplied about parameters like ethnicity, gender, positive family history, age and subtype of MS; however, clinical parameters estimation was carried out by neurology specialist. All patients underwent thorough neurological examination and routine laboratory tests. All were followed up and clinically evaluated at regular intervals in the MS clinic.
Two hundred unrelated healthy individuals, without any autoimmune disease and familial history of MS, were enrolled as control group that came to the Shafa hospital for routine laboratory analysis. Control group was selected by group matching to compare with the patient series. The groups of controls were matched to patients on characteristic such as gender and race; Controls were originally from the same geographical region and were matched with patients in ethnicity. Peripheral blood samples were collected from patients and controls in EDTA tubes. The control participants were also informed about our study and completed a questionnaire form.
Salting out method was used for DNA extraction. Electrophoresis and NanoDrop methods were also applied for assessing the quality and quantity of genomic DNA; so several random extracted genomes were selected for this purpose.
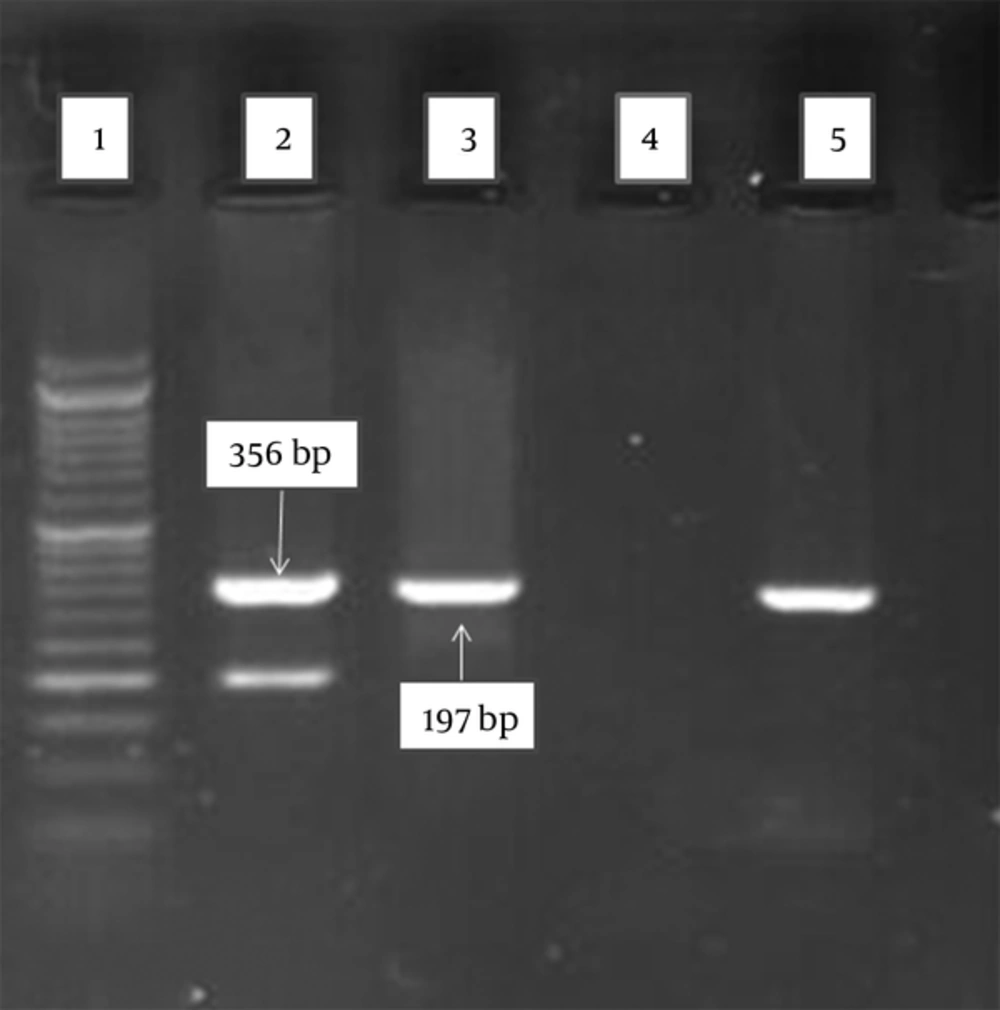
Typing of HLA-DRB1*1501 was analyzed by polymerase chain reaction amplification with sequence-specific primed PCR (PCR-SSP) method and was repeated if discordant results were achieved. The sequences of primers used to amplify the DRBI*1501 were designed using the IMGT/HLA database (http://www.ebi.ac.uk/) and checked out in NCBI/blast (www.ncbi.nlm.nih.gov). The sequences of the forward and reverse primers for exon 2 of DRB1*1501 were 5′-TCCTGTGGCAGCCTAAGAG-3′ and 5′-CCGCGCCTGCTCCAGGAT-3′, respectively. The allele is assigned by the presence of specific PCR product. The size of PCR product may be helpful in the interpretation of the results and it was 197 bp band.
Each PCR-SSP reaction is deemed to have worked if the internal control amplification is observed. Myelin oligodendrocyte glycoprotein (MOG) gene was used as internal control for primers which means in ideal PCR condition, 356 bp band must be appeared. The primers sequences for amplification of internal control were designed by web primer design program, batchprimer3, is accessible at (http://probes.pw.usda.gov/batchprimer3/). They were also aligned in ncbi/blast. The primer sequences were as follow: forward: 5′-GGGACCAATTCTGTGTCACC-3′ and reverse: 5′-TGAACCCAGAAGTCACTCACA-3′.
Finally, the findings were validated by sequencing some samples randomly.
The frequency of the HLA-DRB1*1501 was determined as percentage.
The frequencies of the mentioned allele were compared between the patients and control group using SPSS 16 statistical software and the chi square test. Statistical significance was defined by P value of less than 0.05.
3. Results
Two hundred patients with MS (159 females and 41 males) and 200 healthy sex-matched individuals (144 females and 56 males) were investigated in this study and DRB1*1501 allele was evaluated among them.
The characteristics of the MS patients and control are summarized in Table 1.
| General Information | Controls | MS Patients |
|---|---|---|
| Total individuals, n | 200 | 200 |
| Male | 56 (28) | 41 (20.5) |
| Female | 144 (72) | 159 (79.5) |
| Persians | 116 (58) | 112 (56) |
| Arabs | 84 (42) | 88 (44) |
| Mean age | 57.9 ± 6.7 | 31.16 ± 7.9 |
| Disease course | ||
| RRMS | - | 175 (87.5) |
| SPMS | - | 5 (2.5) |
| PPMS | - | 5 (2.5) |
| PRMS | - | 1 (0.5) |
| CIS | - | 14 (7) |
General Information of Patients and Controlsa
The mean age of patients was 31.16 ± 7.9.
MS disease type (relapsing-remitting, secondary-progressive, relapsing-progressive and primary-progressive) and EDSS score were recorded at date of clinical report. EDSS was evaluated in range of 0 - 10 and most of them had EDSS less than 3.5.
Fourteen (7%) patients had positive family history of MS.
HLA-DRB1*1501 allele was investigated in 200 MS patients and compared with 200 unrelated healthy individuals (Table 2); results showed that the frequency of HLA-DRB1*1501 allele had increased significantly among patients compared with control group statistically (41.5% vs. 22.81%, P < 0.001, OR = 2.400 [95% CI = 1.561 - 3.690]). Gel electrophoresis is shown in Figure 1.
Also the correlation of HLA-DRB1*1501 allele with MS in females and males was analyzed, separately. As it is shown in Table 3, there was no association between this allele and any of the gender. Moreover, no significant correlation was demonstrated between the mentioned allele with type of disease and EDSS, although a significant positive association between DRB1*1501 in Arab and Persian patients were observed, as shown in Table 3.
4. Discussion
Multiple sclerosis has a major heritable component. The aim of present study was to assess the role of HLA-DRB1*1501 allele that are known to influence on MS susceptibility. Our data provide some indications that support the related studies which were already published [11].
Association and linkage disequilibrium studies have confirmed the role of MHC II region, especially HLA-DRB5*0101-HLA-DRB1*1501-HLA-DQA1*0102-HLA-DQB1*0602 haplotypes, which dominate genetic contribution to MS risk. In most studies that have been performed in European populations, the frequency of HLA-DRB1*1501 in patients have been more than that of the controls [12-18]. In non-European populations, such as Brazilian and African-Americans, also the association of HLA-DRB1*1501 and MS has been shown [19, 20]. In some Asian populations such as Turks, Ashkenazi Jews and Indian also the association of the allele and MS has been observed [21-23]; though in a small number of studies in non-European populations such as Chinese, African-Americans, and Afro-Brazilians, researchers found slightly lower prevalence of DRB1*1501 in MS patients than in controls and/or low frequencies of this allele (< 10%) in both patients and controls [24-26].
Moreover, in Iran, as an Asian country, six studies have been carried out in this regard until now. The results of the current study are similar to four of them [5-7, 9] but different from one of them [8]. However, it should be noted that our allelic frequency was more than most of these studies in Iran and this may be due to different genetic pools and ethnic diversity in Khuzestan province.
We also conducted a PUBMED database survey and reviewed some of the most important studies ever carried out on the frequency of HLA-DRB1*1501, in Table 4. As it is shown in Table 4 the maximum and minimum allelic frequencies in patients have been found in Sweden and Mexico respectively until now [18, 27]; thus the greatest association and the smallest p value have been observed in Sweden [18]. The frequency of HLA-DRB1*1501 allele was 41.5% in our population that is almost similar to that of Spain with allelic frequency of 45.9% in MS population and nearly in line with the study was conducted by Ghabaee et al. which frequency of the allele 46% was reported [5, 15].
| Country (Population) | DRB1*1501 Frequency | P Value | OR | (95% CI) | Ref | |
|---|---|---|---|---|---|---|
| Patients, % | Controls, % | |||||
| Brazilian | 21 | 8.85 | 0.0009 | 2.5343 | (1.43 - 4.46) | [19] |
| Greek | 34 | 11 | 0.00015 | - | - | [14] |
| Mexican | 2.94 | 4.62 | NS | - | - | [26] |
| Swede | 61 | 31 | < 0.0001 | 3.5 | (2.7 - 4.4) | [18] |
| Portuguese | 29.8 | 19.9 | 0.008 | 1.72 | (15 - 2.56) | [13] |
| Spanish | 38.6 | 25.5 | 0.0052 | 1.835 | (1.19 - 2.816) | [16] |
| Spaniards/Basque | 45.9 | 34.1 | * | 1.364 | (1.10 - 1.68) | [15] |
| Australian | 57 | 30 | 7 × 10 - 45 | 2.74 | (2.36 - 3.17) | [28] |
| French | 27 | 12 | 0.0001 | 2.79 | (2.1 - 3.89) | [12] |
| Chinese | 18.3 | 21.1 | 0.837 | 0.84 | (0.37 - 1.91) | [24] |
| Turks | 28.2 | 13.9 | 0.02 | 2.4 | (1.2 - 5.0) | [21] |
| Iranians | 46 | 20 | 0.0006 | - | - | [5] |
| 12.5 | 5.5 | 0.0002 | 2.429 | (1.65 - 3.5) | [6] | |
| 13.3 | 4.5 | 0.001 | 3.203 | (1.57 - 6.51) | [7] | |
| 20.8 | 13.5 | 0.24 | 1.68 | (0.49 - 5.42) | [8] | |
| 46 | 34 | 0.0006 | 1.7 | (1.2 - 2.3) | [9] | |
| 41.5 | 22.8 | < 0.001 | 2.400 | (1.56 - 3.69) | ** | |
HLA DRB1*1501 Frequency in MS Patients and Control Group in Different Populationa
Based on the recent study that was carried out by Sharafaddinzadeh et al. MS incidence and prevalence are less among Arabs comparing Persian population in Khuzestan province [29], that is because we calculated the possible association between HLA-DRB1*1501 with Persians and Arabs, separately. According to frequency of the mentioned allele in Persians and Arabs population, significant association in either Persians or Arabs comparing control group was observed. Some association studies have been performed in this regard. A study carried out in Jordan and compared DR1 allele in Palestinians and Jordanians patients with controls; although in mentioned study just the type of HLA was survey, an significant association of HLA-DR1 in patients compared with healthy controls was observed [30]. Moreover, another study confirmed that frequency distribution of HLA-DR epitope in Jordanian Arabs that were clinically defined as MS patients was more than controls and significant association was found [31]. In the Israeli study, HLA alleles was surveyed in Muslim and Christian Arabs, separately; although the DRB1*1501 allele was nominally associated with MS only in the Christian Arab group, no association with Muslim cohort was found [32]. Our finding with regard to Arabs is also similar to the mentioned studies. Hensiek et al. confirmed that HLA-DR15 is associated with female sex in patients with multiple sclerosis [33]; although the results of this study indicate no association between the DRB1*1501 and gender. This result was in line with another study in Greece [14].
There are some studies that showed association between the allele and type of disease [17, 22]. In the Australian study association of HLA-DRB1*1501 with clinical course of multiple sclerosis patients was proved [28]. Even though in this present study no statistically significant differences between DRB1*1501-positive and negative patients were observed according to disease clinical course like some others studies [14].
Logistic regression analysis failed to show any association between HLA-DRB1*1501 and EDSS of MS; EDSS of MS in our sample of patients were similar to features published in observational studies of MS [7, 9, 14]. This suggests that DRB1*1501 may be exerts a susceptibility rather than disease modifying effect in MS.
In summary, the aim of present study is to reveal a part of genetic profile of MS patients in Khuzestan province. The recruited patients originated from south west of Iran. Our results are in line with most of the other previous studies in different ethnic groups in Iran [5-7, 9] and European populations [12-15, 25]. So we can conclude that HLA-DRB1*1501 can be considered as a strong genetic risk factor for MS in our population. For achieving more documented data, it is suggested to type this allele in MS population in other province, specially, in regions considered as high risk for MS.