1. Background
Oral candidiasis is an opportunistic infection of the oral cavity. It is common and underdiagnosed among the elderly, particularly in those who wear dentures and in many cases is avoidable with a good mouth care regimen [1]. Candida albicans often appear in various clinical forms in the individuals with ground disorders. The yeast as a part of normal flora found in humans and animals is able to transfer from one host to another and generates Candidiasis [2]. Oral candidiasis is the most common human fungal infection especially in early and later life. Systemic factors extremes of life predispose to infection because of reduced immunity drugs such as broad spectrum antibiotics alter the local oral flora creating a suitable environment for Candida to proliferate. The normal oral flora is restored once the antibiotics are discontinued [3]. C. albicans is a normal commensal of the mouth and generally causes no problems in healthy people. Overgrowth of candida, however, can lead to local discomfort, an altered taste sensation, dysphagia from oesophageal overgrowth resulting in poor nutrition, slow recovery, and prolonged hospital stay [4]. Various agents such as viscosity, constant tangency, dimorphism, tube germ formation, susceptibility, photo type deformity, intervention with host system, synergism with bacteria, and generating hydrolyzes with other metabolites are hinted as Candidaalbicans virulence agents [5]. Candida albicans as a normal co-exist in mouth cavity is found in 30 - 60 percent and sometimes more than 75% of healthy people [6, 7]. Susceptible agents such as metabolic, nutritional, and mechanical and hospital agents, using denture, smoking, Immunosuppression, and getting vast range of antibacterial treatments can induce Oral Candidiasis, therefore; this disease may be considered as clinical indicator for identifying the presence of susceptible conditions for the individual to catch the disease [8]. Nanoparticles are attributed to a group of atoms with the size of 1 - 100 nanometers [9, 10]. Among nanoparticles, gold nanoparticles attract the researchers’ attention for having specific chemo-physical features [11-17]. Ninane found that 15% - 60% of people with malignancies will develop oral candidiasis while they are immunosuppressed [18]. One of the mineral oxides that has been recently used is Titanium dioxide. It’s used in paint manufacturing and covering texture industries, but its tiny nanoparticles for having extraordinary and unique features (such as electronica, light and photo cabalistic features) have found many applications. This substance is used in refining, disinfections and decolorization, making specific ceramic, demolishing cancer cells, making photo catalyst, paper manufacturing, health care and cosmetics production, providing the protecting cover against ultra-violet and brightening [19, 20]. There can exist about 300 - 400 different species of micro-organism including 20 species of Candida in oral cavity as normal flora [21]. Applying dioxide titanium Nano particles has photo catalyst feature. As you know photo catalyst is a material that in light radiation resulted into chemical reaction but itself never changes and just provide proper conditions to perform a reaction [22, 23]. Titanium dioxide is in the Nano metrical size of an ideal photo catalyst and the most important reason for the presence of this feature is the ability of absorbing ultra- violet rays [24-29].
2. Objectives
This review is to give the reader a contemporary overview of oral candidosis, the organisms involved, and the management strategies that are currently employed or could be utilised in the future. The problem has been compounded by the emergence of Candida species other than C. albicans that have inherent resistance against traditional antifungals. The aim of this study was to compare the antifungal effects of gold nanoparticles and dioxide titanium nanoparticles on patients with oral Candidiasis patients.
3. Methods
This experimental study has been done in Isfahan city totally with 56 numbers of patients suffering from Candidiasis in groups of different ages from hospitals and laboratories. This study uses standard Candida albicans A90029 provided in health education and research center in Isfahan as the control. Taking a history followed by a thorough examination of the mouth, looking at the soft and hard palate, and examining the buccal mucosa in those wearing dentures after they have been removed are usually good starting points. Acute atrophic and chronic hyperplastic forms may mimic other lesions and a biopsy is recommended in addition to empirical therapy to rule out more serious lesions.
• Detection of Candida spp;
• Quickly Swap is put in sterile tube containing physiologic serum with chloramphenicol 500 mg in 1 mL and transferred to the laboratory;
• Preparing slides directly.
Taken samples are put between lam and lamel adding a drop of 10% KOH if necessary provided in order to develop, it is stained by Giemsa or methylene blue methods and studied under the microscope. Among the samples of microscopic tests, the single cell, little, oval-shaped with thin walls, germinated or non-germinated with missile cords cells and the cells with sprout tube or false mycelium are observed and used for the next experiments.
• The phenomenon of creating a germ tube;
• For this purpose, a colony of fungus was mixed with one milliliter of human serum 3 - 4 hours was placed at 37°C. After 72 hours prepared suspension added in slide and put slide under the microscope and was investigated germ tube.
• Create chlamydocanidia in corn meal agar;
• In order to detect chlamydocanidia used corn meal agar medium + tween 80 (Merck, Germany). The plates were inoculated for 24 to 72 hours at room temperature. After a time, a place of inoculation was examined under the microscope;
• Culture in chrome Candida agar.
In order to detect Candida species, culture CHROM Candida agar.one colony of candidas culture medium was cultured in CHROM Candida agar and was placed at 37°C. After a 48 to 72 hours color of colonies on culture media were created. Colonies of Candida albicans is the indicator light green.
3.1. Serial Dilution of Yeast Suspension
After the diagnosis of Candida albicans by the above-mentioned methods, some of the colonies of Candidaalbicans in sterile saline mix and the Candida concentrations prepared with using McFarland standard solutions and spectrophotometer To prepare a bacterial suspension in sterile saline suspension of fungal samples till 0.5 McFarland was prepared.
3.2. Preparation of Standard Dose of Candida albicans Suspension
After preparation McFarland solutions series of tubes 0.5 number of candidates dissolved in saline concentration was used, so that the saline content of the candidate’s turbidity using a spectrophotometer therefore diluting with saline containing Candida, it’s finally light absorption in the wavelength to 0.08 - 0.1 requited. In this case, approximately in each mL of saline solution containing the yeast of CFU/mL 1.5 × 108, which is a yeast cell concentration, is standard antifungal effects. Properties of gold nanoparticles: 200 mL of colloidal gold nanoparticles in colloidal solution at 10 nm and a concentration of 100 ppm to a spherical shape and 100 mL of gold nanoparticles bought from Neutrino Company in Tehran and dilutions (5, 10, 25, 50) ppm it was prepared. 300 mL, dioxide titanium Nano particles from the Spanish company neutrino Tehran that provides the nanoparticles, the specifications: diameter of 10 - 15 nm, the purity of 9/99 percent concentration of 1000 ppm was purchased.
3.3. Diffusion Method
56 isolates of Candida albicans (the biopsy place isolated from mouth and The samples using swabs Sterile by pulling on at least three points of the mucosa The samples were prepared from mouth dorsal and surface of the tongue, mucous atrium oral between species and molars.), sterile cotton swab on the surface of the agar medium sabouraud was growing steadily. 100 microliters of different concentrations of gold nanoparticles produced separately added to each well. The 100 mL concentrations of 5, 10, 25 and 50 ppm prepared from gold nanoparticles were added to each well. Sterile water as negative control and pharmaceutical disc 25 mg fluconazole disk was used as a positive control. The inhibition zone diameter was measured in millimeters. Also in the medium sabouraud Swab sterile agar was steadily growing. The end of the Pasteur pipette, pit to a diameter of 6 mm was created in vitro. 100 mL of dilution of 10, 100, 300 and 500 ppm prepared from Dioxide Titanium Nano particles was added to each well. Sterile water as negative control and the drug fluconazole 25 mg hard disk (Himedia India Company) was used as a positive control. The experiment was repeated three times for each sample and inhibition zone diameter in milimeter after 48 hours at 37°C was measured.
3.4. Microdilution Method
MIC material used was determined by microdilution method M27-A [30]. In these methods, under aseptic conditions, For Nano gold particles: 100 mL of dextrose broth sabouraud environment sterile microplate was added to each of 96 wells. Then were added 100 ml of dilution and 100 ppm of gold nanoparticle then were added dilution made with gold nanoparticles 5, 10, 25 and 50 ppm. And For dioxide titanium: were added 100 mL of dilution of 100 ppm nanoparticles of dioxide titanium that prepared in (10, 100, 300, 500) ppm concentrations. Houses 10 and 11 in the microplate related to positive and negative control is. In the end, all the wells 10 mL of fungal suspension of each isolate were added. Microplates are incubated for 24 hours at 37°C. After incubation, the wells were checked for turbidity. Staining that growth was not in it was considered as the MIC. From two wells to wells where no growth was, on sabouraud dextrose agar medium to obtain the MFC [31]. Finally, using SPSS-15 and analyzed and evaluated mean differences plotted charts and graphs with Excel 2010 software. Results significant at P < 0.001 investigated. The positive control was disk of fluconazole.
4. Results
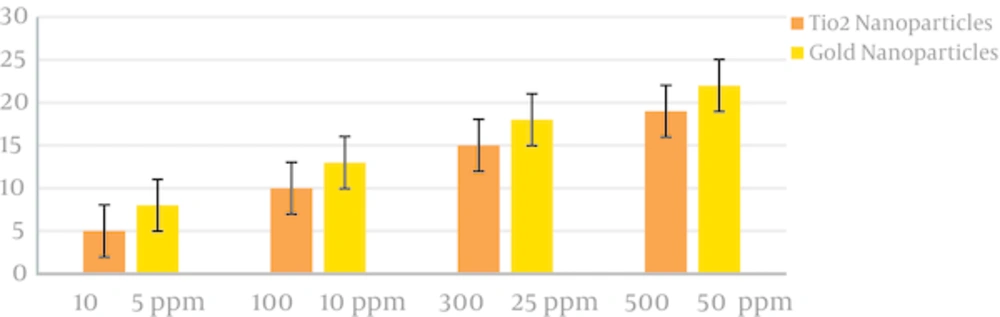
Diffusion method results from the impact of the gold nanoparticles on samples of C. albicans. Figure 1 shows the diameter of the inhibition of Candida albicans isolates the impact of the gold nanoparticles on the disease. The results published in the well showed that the diameter of fungal growth inhibition increased with increasing concentrations of gold nanoparticles. The diameter of the growth of gold nanoparticles 10 nm on isolates, zero to 22 mm and the diameter of the growth of nanoparticles of titanium dioxide was zero to 19 mm. Table 1. Mean diameter of four levels of growth the study shows. The mean diameter of inhibition at concentrations of 50 ppm of gold nanoparticles larger than the average diameter of the inhibition at concentrations of 500 ppm of dioxide titanium nanoparticles and gold nanoparticles compared to Dioxide Titanium Nano particles, in different concentrations, the inhibition zone diameter was higher. (The significance level was almost zero) and (P < 0.001). Most average inhibition zone diameter gold nanoparticles at concentrations (5, 10, 25, 50) ppm, respectively, 8, 13, 18.22 mm. for the dioxide titanium nanoparticles concentrations (10, 100, 300, 500) ppm, respectively, was 5.11. 15.19 mm. Figures 1 and 2 concentrations (5, 25.10 and 50) ppm of gold nanoparticles and concentration (10, 100, 300, 500) ppm of Dioxide Titanium nanoparticles is clearly shown that gold nanoparticles in Most concentrations have different growth inhibitory zone. The results showed that gold nanoparticles have a good anticandidial effects and can be used to treat infections of Candida albicans. The values of inhibition zone diameter of fluconazole as expected as a positive control was 18 mm.
| Gold Nanoparticles Concentration | Mean ± SD | Dioxide Titanium Nanoparticles concentration | Mean ± SD |
|---|---|---|---|
| 5 | 7.34 ± 5.28 | 10 | 3.51 ± 1.98 |
| 10 | 11.76 ± 8.31 | 100 | 9.28 ± 5.68 |
| 25 | 16.28 ± 12.70 | 300 | 14.76 ± 10.69 |
| 50 | 21.92 ± 16.38 | 500 | 18.95 ± 13.55 |
4.1. MBC and MIC Results
On samples isolated from patient’s oral candidiasis with microdilution method Gold nanoparticle samples were determined according to this method. The minimum inhibitory concentration (MIC) and the minimum fungicidal concentration (MFC) of gold nanoparticles were in turn 5.65 ± 8.37 and 45.56 ± 23.78 and for dioxide titanium Nano particles were in turn 19.31 ± 17.53 and 34.20 ± 21.68. Based on the attained results Anti-fungal activity of gold nanoparticles was depended on concentration.
| MIC | MFC | |
|---|---|---|
| Gold nanoparticles | 5.65 ± 8.37 | 45.56 ± 23.78 |
| Dioxide titanium nanoparticles | 19.31 ± 17.53 | 34.20 ± 21.68 |
5. Discussion
According the result of this study, using gold nanoparticles with 10 nanometer diameters have high antifungal effect on oral candidiasis and its function has been proved. In current study halting effect of gold nanoparticles on micro-organisms experimented in different densities was observed. The mean diameter of inhibition at concentrations of 50 ppm of gold nanoparticles larger than the average diameter of the inhibition at concentrations of 500 ppm of dioxide titanium nanoparticles and gold nanoparticles compared to dioxide titanium Nano particles, in different concentrations, the inhibition zone diameter was higher. Based on the attained results anti-fungal activity of gold nanoparticles was depended on concentration.
Oral candidiasis is a common opportunistic infection of the oral cavity caused by an overgrowth of Candida species, the commonest being Candida albicans. The incidence varies depending on age and certain predisposing factors. There are three broad groupings consisting of acute candidiasis, chronic candidiasis, and angular cheilitis. Risk factors include impaired salivary gland function, drugs, dentures, high carbohydrate diet, and extremes of life, smoking, diabetes mellitus, Cushing’s syndrome, malignancies, and immunosuppressive conditions. Management involves taking a history, an examination, and appropriate antifungal treatment with a few requiring samples to be taken for laboratory analysis. In certain high risk groups antifungal prophylaxis reduces the incidence and severity of infections. The prognosis is good in the great majority of cases [7]. Nirmala Grace et al. [9] studied the influence of antifungal medication covered by nanoparticle in different dilution with philoconazol on the growth of Aspergillus flavous fungi. The best activity of antifungi in floconasal covered by gold 40 ppm was observed. The attained diameter of corona was 12mm but in our research 50 ppm of gold nanoparticles have the best activity of antifungi. As the high potential of gold nanoparticles in effecting on clinical isolated Candida albicans, their possible application in disease treatment can be considered [10]. Nanoparticles can perform as antibacterial and antifungal. this effect is based on linking to cell membrane that cause the alternation and structure deficiency and the function of cell like penetrating feature, and also by producing cracks and holes influencing on enzymes of respiratory chain causes cell death [10, 11]. In Zawrah et al. [11] research in 2011 anti-microbial activities of gold nanoparticle were studied. Gold nanoparticles attach to Candida albicans membrane and cause observable damage in cells with a complete destruction of flagella. Jebali et al. [12] also studied Anti-fungal ability of spiral gold nanoparticles on Candida albicans isolated and also standard Candida albicans in laboratory condition. Chwalibog et al. [13] also indicated that the best anti-fungal activities of gold nanoparticle are against Aspergillus niger and Candida albicans. Anti-microbial ability of gold nanoparticles might be because of their very small size of 9 - 19 nanometers. This feature causes them to attach easier to micro-organism membrane and cause their destruction. Gold nanoparticles are able to keep their shape and size in solvent. Treatment process by gold nanoparticles can reduce the length of treatment course and implications caused by medication in which these results is similar with our findings in my research the size of gold nanoparticles was 10 nm. Chwaliboget stated that metal nanoparticles of gold nanoparticle, silver nanoparticle, platinum are harmful fungi and bacteria and by using electronic microscope show the morphological changes resulted from interaction between micro-organisms and nanoparticles. This interaction causes damage of fungal cells. Silver nanoparticles attach to microbial cell membrane and this attachment cause structure change and cell damage and specially resulted in cell bioactivities such as penetrability and the effect on enzyme activities of respiratory chain and eventually end to cell death. Silver nanoparticles halter yeast growth, and have antifungal activities against different species of Candida albicans. They also indicate that gold nanoparticle and floconazol covered by gold at least show different subjugating dilution against Candida albicans, Aspergillus niger and Aspergillus flavous. Gold nanoparticles destroy cell wall and cytoplasmic membrane Candida albicans and cause to release homogenized substance then attach to filament substance and cause to disintegrate of cell [13, 14]. Wani et al. [17] Indicated that gold nanoparticles with haltering ATPase activities in Candida albicans yeasts do their own anti-fungal activities against them. Ninane [18] studied the effect of light photo catalyst of titanium dioxide on Candida albicans fungi and Phozarium solani after 8 hours radiation of ultra-violet ray in 300 nanometer in 200 w/m, reported the decrease of wave length 400 at least 410 g in the life of fungal cells. Battin et al. [19] with the study of titanium dioxide nanostructure and its effect on microbial biofilm cells indicated that demolishing cell membrane in freelance cells is more than biofilm cells. Seven et al. studying antimicrobial features of dioxide titanium Nano particles and zinc dioxide state that the effect of them on cellular suspension of Candida albicans, Saccharomyces cerevisiae and Aspergillus niger fungi and E. coli, Pseudomonas aeruginosa and Staphylococcus aureus bacteria destroyed. Three species of bacteria destroyed in 40 minutes while the fungal species demolished in 120 minutes under the same conditions with sodium lamp radiation 400w [20]. The results showed that gold nanoparticles have a good anticandidial effects and can be used to treat infections of Candida, it is recommended that further research considered the effects of different infections candidiasis in In vivo condition.