1. Background
The outburst of new pathogenic agents and development of multidrug resistant strains are claiming millions of lives of people around the world by bacterial infections. The neutralization of biocide action of antibiotic molecules is achievable with progressed efficient attitudes from the pathogens (1). As a result, multidrug-resistant bacteria are sensitive against only a few abundant available antibiotics (1). Therefore, search for new effective antibiotics is very important. Researchers and pharmaceutical companies are therefore searching to achieve this goal. Recently, nano-particles have been demonstrated as effective antimicrobial agents (1, 2).
The production, manipulation, and use of matter in the size of 1 to 100 nm is known as nanotechnology. Elements, including gold, magnesium (3), alginate (4), titanium, zinc, copper (5) and silver are used to make nanoparticles. Appropriate anti-microbial activity of silver nanoparticles has led to their consideration for intensive research (6); therefore, there is an urgent need to synthesize nanoscale materials that are safe and reliable in the environment. The cooperation between nanotechnology and other sciences, such as materials chemistry and biotechnology, is imperative to reformulate new procedures in the synthesis of nanomaterials, with precise control over their shape and size (7). One of these new synthetic paths is microbial biosynthesis for the production of nanoparticles by microorganisms (fungi, bacteria, and algae) (7).
In nanoparticle synthesis, easy handling, affordability with simple nutrients and wide wall-binding capacity of filamentous fungi are advantages over bacteria (8). In this case, Fusarium oxysporum is a fungal species which has several reports (9). Fusarium acuminatum (10), Fusarium semitectum (11), Aspergillus niger (12), Aspergillus flavus (13), and Penicillium fellutanum (14) were also recently shown to be applicable for the production of nanoparticles. Endophytic fungi, in addition to their unique properties, can be used to produce nanoparticles. However, there are very few reports in this context. A limited number of endophytic fungi, such as Colletotrichum sp. (15), Aspergillus clavatus (AzS-275) (7), and Penicillium sp. (16) have been studied in this case. In this study, the researchers attempted to screen for endophytic fungi from Taxus baccata L. (Iranian Yew) that have the ability of extracellular biosynthesis of silver nanoparticles.
2. Methods
2.1. Isolation of Endophytic Fungi
Sampling of T. baccata L. (Iranian Yew) was done from the forests at the valley of Zarrin, Aliabad-e Katul, Golestan province. Barks from the stem and branches of trees over 100 years old were selected for sampling and transported to the laboratory without delay. The barks were washed under running tap water and cut to smaller pieces. Small pieces were surface sterilized by 70% ethanol for one minute and a disinfectant for four minutes (0.5% sodium hypochlorite). Subsequently, these pieces were washed three times with distilled water. Then, they were allowed to become completely dried under sterile conditions. After removing the outer bark with a disinfected cutting edge, the inner bark was placed on a petri dish containing PDA medium supplemented with 500 mg/L chloramphenicol. After a long day of incubation at 28°C, fungi were grown from the bark pieces. At this stage, fungi purification was performed by the hyphal tip method. In this way, hyphal tips were removed from different fungal colonies and placed on new PDA medium, and incubated at 25°C for at least 10 days. This procedure was repeated three times to ensure a perfect purity.
2.2. Mycosynthesis of Silver Nanoparticles
Purified endophytic fungi were screened for extracellular synthesis of silver nanoparticles. For this purpose, they were incubated in MGYP broth at 28°C. Biomass was filtered after 72 hours of incubation, and medium components were removed by washing with distilled water. Biomass was incubated in the Erlenmeyer flasks, including 100 mL of distilled water for 48 hours at 28°C. The biomass was then filtered. In order to achieve reduction, 1 mM of silver nitrate (AgNO3) solution was mixed with fungal filtrate as a reducing agent. The color change of the mixture indicated the formation of silver nanoparticles. UV-vis spectrum of the reaction solution finally confirmed this formation.
2.3. Characterization of Silver Nanoparticles
The solution containing silver nanoparticles was centrifuged at 10,000 rpm for 15 minutes to concentrate nanoparticles in pellet forms. Sterile distilled water was added to these pellets for re-suspension and again centrifuged for 10 minutes at 10,000 rpm. In this step, pellets were dried at room temperature for IR analysis. The FTIR spectrum recorded the possible biomolecules involved in the synthesis and stabilization of nanoparticles. To determine the shape and size of nanoparticles, the characterization of AgNPs was carried out by particle size analyzer (VASCO-Cordouan Technologies) and TEM analysis (model: Leo 912 AB).
2.4. Antibacterial Activity of Silver Nanoparticles
In the agar well diffusion assay, biosynthetic silver nanoparticles were studied against pathogenic bacteria (Bacillus subtillis, Escherichia coli, Pseudomonas aeruginosa, Staphylococcus sp., and Salmonella typhi) for antibacterial activity (17, 18). Wells were loaded with 20, 40, 60, and 80 µL of silver nanoparticles suspension. In order to analysis the zone of inhibition, clear areas around the wells were measured after incubation of the inoculated plates at 37°C for 24 hours.
2.5. Identification of the Fungal Isolate Used for Biosynthesis of AgNPs
Genomic DNA was extracted using Doyle J. and Doyle J.L. (1987) CTAB procedure (19) (with little modification). The ITS regions were amplified by PCR, using universal primers ITS1 (5’-TCCGTAGGTGAACCTGCGG-3’) and ITS4 (5’-TCCTCCGCTTATTGATATGC-3’) (20). Amplification conditions were as follows: initial denaturation for three minutes at 95°C followed by 35 cycles of denaturation for one minute at 94°C, primer annealing for 30 seconds at 55°C, extension for one minute at 72°C and a final extension for 20 minutes at 72°C. Analysis of PCR product was done on 1.2% (w/v) agarose gel and amplified DNA was purified by isopropanol precipitation and sequenced (Macrogen, South Korea). To acquire phylogenetic sequences, the sequences associated with endophytic fungi were analyzed using the BLAST algorithm of NCBI database. By using the neighbor-joining method and MEGA software (version 5.0), phylogenetic analysis was performed.
3. Results and Discussion
3.1. Extracellular Synthesis of Silver Nano-Particles
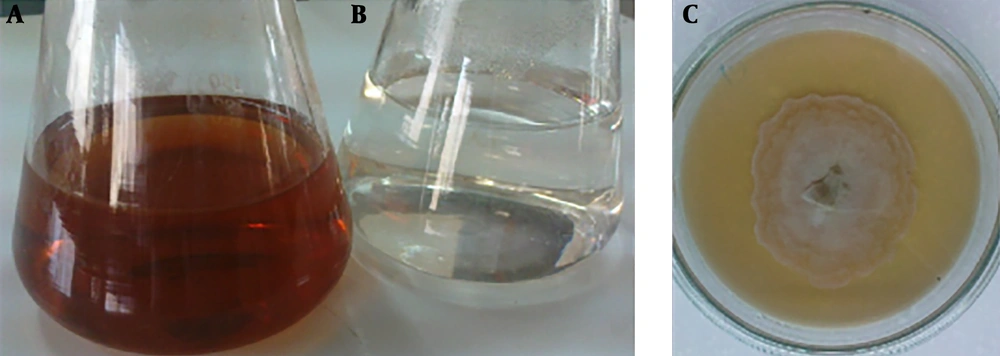
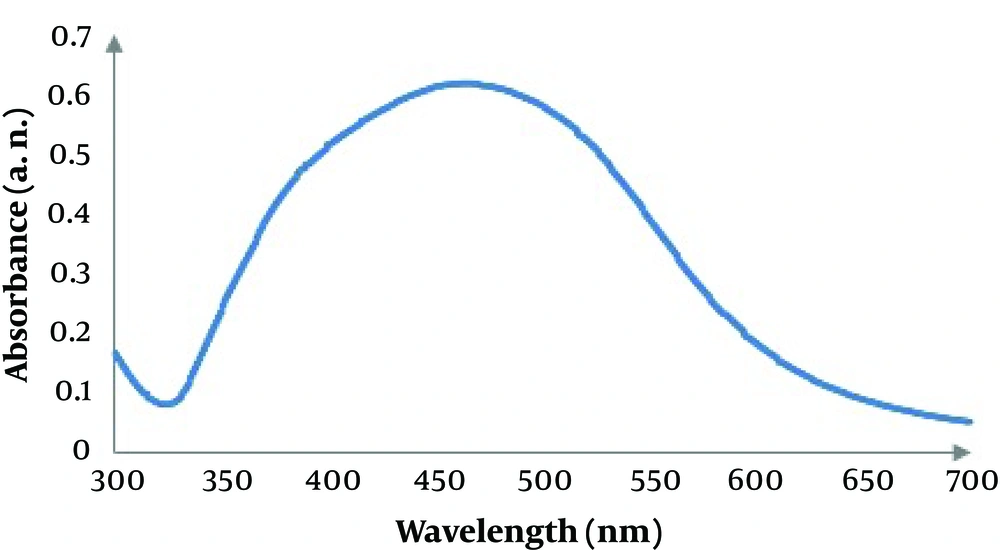
Screening for biosynthesis of AgNPs with seven endophytic fungi (ETB1, ETB2, ETB3, ETB4, ETB5, ETB6 and ETB7) isolated from T. baccata L. (Iranian Yew) was done, and only one of them (ETB3) showed as a manufacturer of nanoparticles. The color of the reaction mixture changed from colorless to brown (Figure 1) after 24 hours of mixing the fungal filtrate with 1 mM aqueous solution of AgNO3. In fact, the brown color appeared with confination of the metallic silver nanoparticles because of the appearance and collective oscillation of the conduction electrons (9). Absorption spectra confirmed the formation of the reduced AgNPs in the colloidal solution with absorbance peak at 460 nm (Figure 2). This broadened peak indicated the polydispersed particles.
3.2. Particle Size Analysis
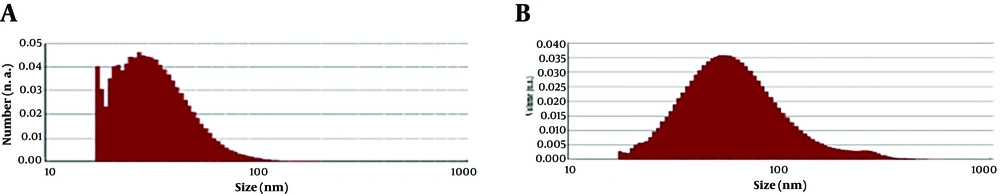
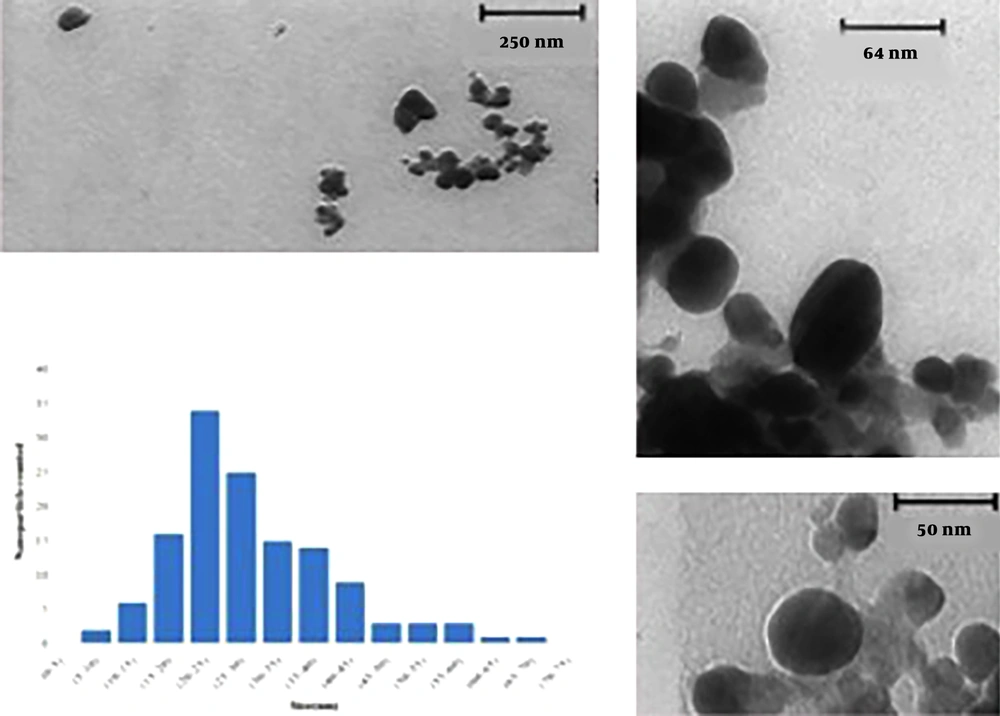
Result of particle size analysis is shown in Figure 3. In this analysis, D mean number was 33.54 and highest frequency of the nanoparticles was with a diameter of 26.9 nm in the size dispersion by number. D mean volume was also 69.43.
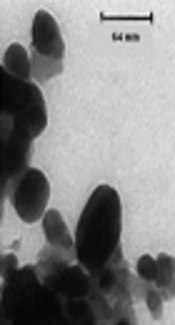
3.3. TEM Analysis
Representative TEM images of silver nanoparticles biosynthesized by the endophytic fungal isolate are shown in Figure 4. The variation was visible in the shape of the nanoparticles, yet most of the nanoparticles were spherical or close to ellipsoidal in the size range from 5 to 70 nm and average size 33.52 nm. In the particle size and TEM analysis, it was also observed that with increasing reaction time, nanoparticles collapsed and formed large aggregates.
3.4. FTIR Analysis
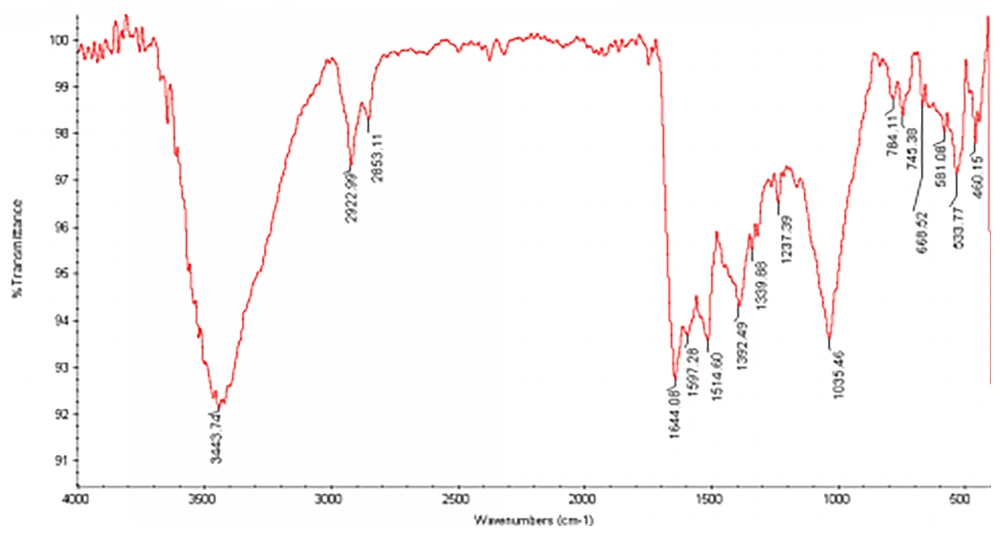
The FTIR study of AgNPs showed major peaks around 1035, 1392, 1514, 1644, 2922 and 3443 cm-1 (Figure 5), demonstrating presence of amides on AgNPs. The assignment of each of these absorptions is given in Table 1. According to the table information, a protein layer in addition to other bio-organic molecules can be formed as reducing agents. Well dispersed nanoparticles can be achieved by the effect of this layer on stabilization (Table 1).
| IR Absorption/(cm1) | Assignment |
|---|---|
| 1035 | C-N stretching vibrations of aliphatic amides |
| 1392 | C-N stretching vibrations of aromatic amines |
| 1514 | Bending vibrations of amide II |
| 1644 | Bending vibrations of amide I |
| 2922 | Corresponding stretching vibration of protein nanoparticles |
| 3443 | Corresponding stretching vibration of protein nanoparticles |
3.5. Antibacterial Activity of Silver Nanoparticles
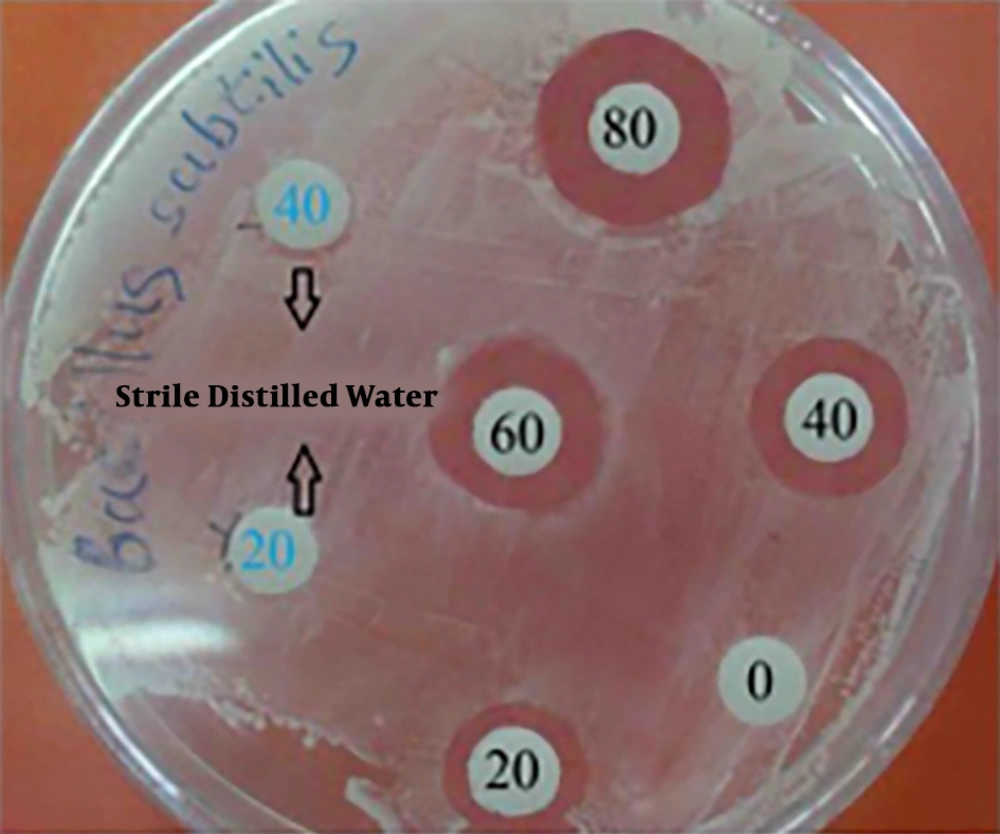
Antimicrobial properties of silver have long been known and are used in the medical field. Despite different assumptions about the mechanism of silver nanoparticle action, its exact mechanism is unknown (21). Silver nanoparticles disrupt cell-wall permeability and cellular respiration since they may attach to the cell wall. Finally, interactions between nanoparticles with phosphorus and sulfur-containing compounds, such as DNA and protein, cause cell death. Silver nanoparticles can enhance their bactericidal activity by releasing silver ions in bacterial cells (22-24). In this study, antimicrobial activity of biosynthesized AgNPs against B. subtillis, P. aeruginosa, E. coli, Staphylococcus sp., and S. typhi were tested, using the agar well diffusion assay method (Table 2). Zone of inhibition varied between 10 mm and 17 mm and its maximum (17 mm) was observed with B. subtillis at 80 µL of silver nanoparticles suspension (Figure 6).
| Pathogenic Bacterial Strains | Zone of Inhibition (mm) | |||
|---|---|---|---|---|
| 20 µL | 40 µL | 60 µL | 80 µL | |
| Bacillus subtillis | 10 | 15 | 16 | 17 |
| Escherichia coli | 10 | 13 | 14 | 15 |
| Staphylococcus sp. | 10 | 12 | 13 | 15 |
| Pseudomonas aeruginosa | 11 | 14 | 14 | 16 |
| Salmonella typhi | 11 | 13 | 14 | 16 |
3.6. Identification of the Fungal Isolates
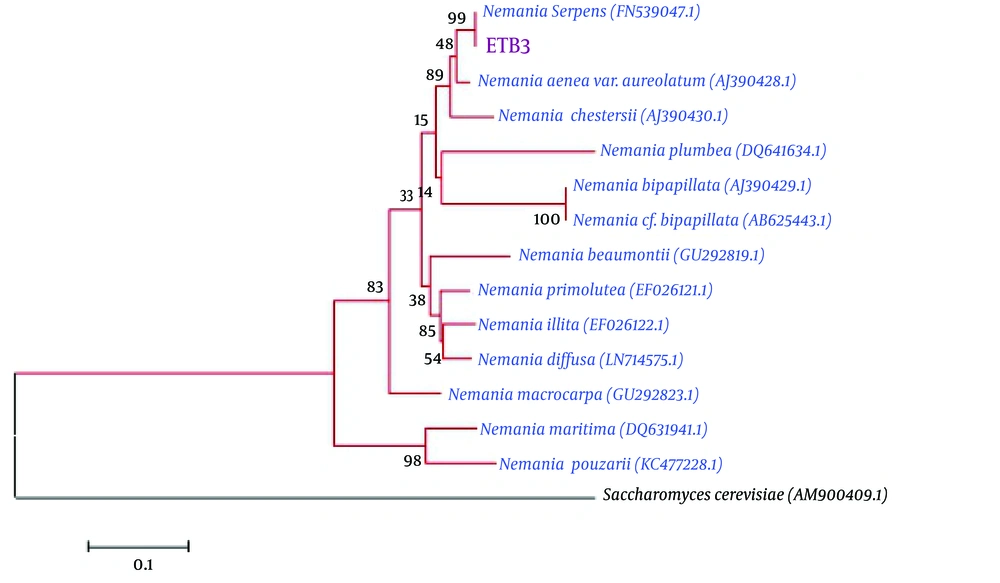
The endophytic fungal isolate belonged to Nemania sp. with ITS sequence analysis, in ~504 bp, which showed 99% similarity to Nemania serpens (FN539047.1). The neighbor homologues sequences of ITS sequences were selected for the multiple sequence alignments, using the ClustalW program in the MEGA 5 software. The neighbor-joining algorithm was used for phylogenetic tree construction with 1000 bootstrap replications (Figure 7).
4. Conclusions
The present study demonstrated the isolation of endophytic fungi from Taxus baccata L. (Iranian Yew) and screened for their efficacy to reduce AgNO3 to AgNPs. Characterization of the synthesized AgNPs was carried out using UV-Vis spectrum, TEM, and FTIR. The particle sizes were in the range of 5 to 70 nm with average size 33.52 nm and the AgNPs displayed promising antibacterial effects, which will be valuable for biomedical applications.