1. Background
Liver diseases are considered one of the most important causes of death in the world [1]. Oxidative stress is known as a mechanism involved in the onset and progress of hepatic damages [2]. The increase in the levels of reactive oxygen and nitrogen species can cause hepatocellular injuries [3].
Unsaturated fatty acids are named according to the location of double bond from the carbon of the terminal methyl called omega carbon. The fatty acids, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) belong to the omega-3 fatty acid category. These acids can't be synthesized by humans and should be included in diet; the main sources of omega-3 are fish oil, marine planktons and oceanic fish [4]. In a study, Wergedahl et al. showed that combination of fish oil (FO) and fish-protein hydrolysate (FPH) reduces plasma cholesterol levels, which are related to their effects on the reduction of HDL cholesterol level, while the total hepatic cholesterol concentration increased, compared to control mice and those receiving FPH and FO alone. The cholesterol reducing effects of combined FPH and FO is related to decreased secretion of low density lipoproteins (LDL) from the liver [5]. The studies of Kim et al. demonstrated that omega-3 fatty acid has protective effects against insulin resistance induced by obesity and liver steatosis. Omega-3 hyperlipidemia induced by diet and fatty liver can be improved through induction of cytochrome CYP7A1 expression and the activity of cholesterol catabolism to bile acids [6]. In a study by Haast et al., a direct correlation was found between Omega-3 fatty acid intake and reduced age-related brain deterioration and damage [7]. Also, Siegel et al. showed that omega-3 fatty acids have beneficial effects on cerebral-cardiovascular diseases [8]. Likewise, fish oil omega-3 emulsion significantly reduces hepatic damage following liver transplantation [9], and has protective effects against liver fibrosis and injuries as well as oxidative stress induced by carbon tetrachloride [10]. Administration of omega-3 fatty acids may reduce infection rate and improve liver function recovery in HBV-associated hepatocellular carcinoma patient after hepatoctomy. This improvement is associated with suppressed production of proinflammatory cytokines in these patients [11].
Thioacetamide (TAA) is an organic compound containing Thiono-sulfur that is used as a fungicide, an organic solvent and a stabilizer of motor oil. In 1984, Hugh and Nelson first reported that TAA is a hepatotoxic agent. A single dose of this agent can produce lobular necrosis sentry in animals, and chronic induction of thioacetamide can lead to liver cirrhosis and carcinoma [12]. The toxic effects of thioacetamide is due to its biological activity exerted through oxidase systems, particularly FAD mono oxygenases and CYP2E1 [13]. Hence, in conjunction with the measurement of hepatic enzymes, thioacetamide can be useful in pharmaceutical research to induce pathological condition, because many blood factors and enzymes are synthesized in hepatocytes and their measurement is a diagnostic criteria for liver function.
2. Objectives
Due to high prevalence of liver disease and the high side effects of current chemotherapies, there is a growing need for a medicine (s) with minimum complications and high effectiveness. In this regard, the use of fish oil omega-3 is most promising, since it has antioxidant and anti-inflammatory properties and shows low side effects. Thus, in this research we tried to examine the possible protective effects of this oil on changes in the levels of gamma glutamyl transferase (GGT), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), serum glutamic oxaloacetic Transaminase (SGOT), serum glutamic pyruvic Transaminase (SGPT), albumin, bilirubin and total protein induced by thioacetamide in pretreated rats.
3. Methods
3.1. Animal Experiments
This was an experimental study. Animals were collected from Fars Razi vaccine and serum research institute. All guidelines for ethical conduct in the care and use of laboratory animals developed by the ministry of health and Medical education were observed in the present study. 42 adult male wistar rats in a weight range of 200 ± 10 g and the age range of 3 - 2.5 months were used. Animals were randomly divided in 6 groups of seven, and kept under standard conditions of 20 - 22°C and light cycle of 12 hours light and 12 hours dark. They had easy access to food and water, and all ethical considerations and animal rights were ensured.
3.2. Experimental Design
Animals were divided into 6 groups and were treated as follow: the control which left untreated and subjected to no stress ; the sham 1 which daily received 0.4 mL/kg olive oil as a solvent supplement of fish oil omega-3; the sham 2 which received a single dose of 150 mg/kg thioacetamide; the experimental groups 1, 2 and 3 received a daily dose of 100, 200 and 300 mg/kg fish oil omega-3 supplements respectively followed by a single dose of 150 mg/kg thioacetamide (Stigma-Aldrich, Switzerland). Fish oil omega-3 was administered orally for 3 months, and thioacetamide was injected interperitonaly at the end of treating period.
3.3. Biochemical Study
As the toxic effects of thioacetamide usually becomes apparent about 2 days after the injection, 48 h after the last injection, animals were anesthetized with ether (Merck, Germany), and blood samples were taken from the heart. These samples were kept under laboratory conditions for 20 minutes; then, centrifuged at 5000 RPM for 15 minutes (Hettich, Germany) [14, 15].
Serum concentrations of various parameters were measured by appropriate methods: SGPT, SGOT and LDH by buffer phosphate method, DGKC and ALP by P-Nitrophenyl phosphate method, AMP and albumen by Bromocresol Green method, total protein by biuret reaction end point method [16], and GGT by enzymatic method (by the kit from Darman Kav, Iran). To measure bilirubin, azo DVD reagents (sulfanilic acid sodium nitrite) were used; they react with bilirubin, producing azo, which is red in alkaline PH. After production, direct bilirubin has a pink color, but after addition of accelerating solution, total bilirubin turns green in alkaline PH [17].
3.4. Histological Tests
After removal, livers were fixed separately in 10% neutral formalin buffer. The dehydration was done by alcohol with different concentrations (from low to high). For clearing, the tissues were placed in two Xylene containers. Then in the infiltration stage, the tissues were soaked in melted paraffin (65°C), each for an hour. In the molding stage leukhardt parts were used. Following histological tissue preparation and producing specimen blocks, 4 - 5 micron thick sections were prepared, stained using hematoxylin-eosin method and studied by light microscope [18].
3.5. Statistical Analysis
The software program SPSS (Version18, Chicago, IL, USA) and statistical ANOVA test were used for data analysis. In order to study statistically significant differences among data, the Tukey HSD test was used and significant average difference was sat at P < 0.05. The serum concentrations of GGT, LDH, ALP, SGPT, SGOT, total protein, albumin and bilirubin are presented as average deviation.
4. Results
The mean serum concentration of SGOT significantly increased in the sham 2 (receiving thioacetamide alone) and experimental groups 2 and 3 (receiving both fish oil omega-3 supplements and thioacetamide) compared with the control and sham 1, while it showed an insignificant reduction in experimental groups 2 and 3 compared with the sham 2 (Table 1). Similarly, the mean serum level of SGPT significantly increased in the sham 2 and experimental group 2 relative to the control and sham 1, whereas significantly decreased in the experimental group 1 (receiving both 100 mg/kg fish oil omega-3 supplement and thioacetamide) compared with the sham 2 (recipient of thioacetamide alone) (P < 0.05). Conversely, in respect to mean concentrations of ALP, there were no significant differences among various experimental groups and the sham 2, nor were any such difference among the sham 2, and control and sham 1 group (Table 1).
| All Group | SGPT, (U/L) | SGOT, (U/L) | ALP, (IU/L) | Bilirubin, (mg/dL) |
|---|---|---|---|---|
| Control | 189.60 ± 10.60a | 83/66 ± 1.17a | 1456.66 ± 54.66 | 0.12 ± 0.015a |
| Sham 1 | 201 ± 6.70a | 88 ± 2.19a | 1418 ± 70.84 | 0.23 ± 0.029 |
| Sham 2 (TAA) | 361 ± 10.01 | 187.50 ± 1.44 | 1173 ± 70.15 | 0.38 ± 0.015 |
| 100 mg/kg omega3+TAA | 234.75 ± 12.61b | 147.25 ± 10.75 | 1418 ± 52.47 | 0.06 ± 0.013b |
| 200 mg/kg omega3+TAA | 364.75 ± 12.17c | 201.50 ± 11.96c | 1684.66 ± 70.87 | 0.17 ± 0.010 |
| 300 mg/kg omega3+TAA | 278.75 ± 7.49 | 165.25 ± 13.38c | 1377 ± 88.04 | 0.14 ± 0.026 |
The Effects of Different Amounts of Fish Oil Omega-3 Supplement on Various Biochemical Serum Parameters in Male Rats Poisoned by Thioacetamide
In addition, the mean serum concentration of bilirubin significantly increase in the sham 2 group relative to the control and sham 1 groups, while it showed no significant changes in all experimental groups relative to the control and sham 1 groups, but significantly decreased in all three experimental groups, and the group receiving thioacetamide (P < 0.05). Also, In regard to concentration of total protein, no significant changes were observed between group receiving thioacetamide, and the control and sham 1 group as well as between various experimental groups, and the control, sham 1 and sham 2 groups (Table 2).
| All Group | Total protein (mg/dL) | Albumin (mg/dL) | GGT (U/L) | LDH (U/L) |
|---|---|---|---|---|
| Control | 8.88 ± 0.5 | 4.30 ± 0.03a | 2 ± 0.26a | 554.28 ± 6.58a |
| Sham 1 | 8.70 ± 0.07 | 4.30 ± 0.04a | 2.50 ± 0.22a | 599.66 ± 33.84a |
| Sham 2 (TAA) | 8.56 ± 0.08 | 4.70 ± 0.00 | 4.50 ± 0.28 | 849 ± 0.57 |
| 100 mg/kg omega3+TAA | 8.58 ± 0.05 | 4.40 ± 0.02b | 2.17 ± 0.15b | 962.75 ± 18.17c |
| 200 mg/kg omega3+TAA | 8.53 ± 0.20 | 4.32 ± 0.10b | 2.20 ± 0.18b | 955.25 ± 9.59c |
| 300 mg/kg omega3+TAA | 8.63 ± 0.24 | 4.34 ± 0.10b | 3.75 ± 0.25 | 1022 ± 30.23c |
The Effects of Different Amounts of Fish Oil Omega-3 Supplement on Various Biochemical Serum Parameters in Male Rats Poisoned by Thioacetamide
In contrast, the mean levels of albumen significantly increased in group receiving thioacetamide compared with the control and sham 1 group, whereas it showed no significant changes in all experimental groups relative to the control and sham 1 groups, but a significant decline was seen between all three experimental groups, and the group receiving thioacetamide (Table 2). The mean concentrations of GGT and LDH significantly increased in the sham 2 group compared with the control and sham 1 group. Nevertheless, the mean level of GGT did not show a significant change in all three experimental groups relative to the control and sham 1 group while the mean level of LDH significantly increased in these groups. Conversely, the average concentration of GGT in experimental groups 1 and 2 (receiving 100 and 200 mg/kg fish oil omega-3 supplement and thioacetamide respectively) significantly decreased relative to the sham 2 group whereas no differences was observed in the mean concentration of LDH among all three experimental groups and the sham 2 group (P < 0.05; Table 2).
4.1. Histological Findings
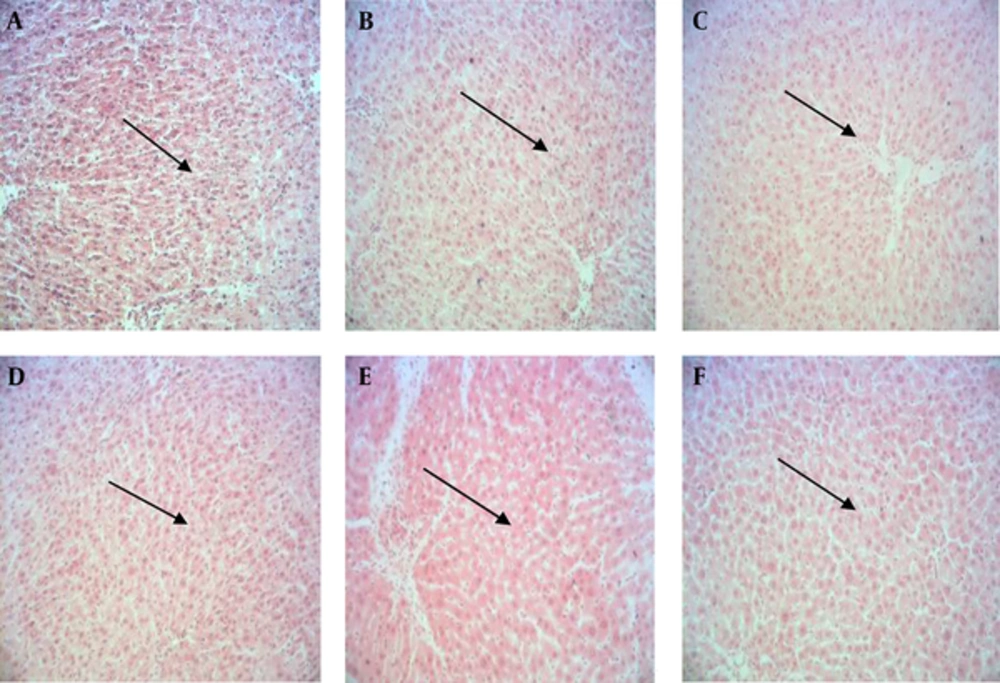
Histological studies of hepatic samples showed normal hepatocytes with protected cytoplasm and marked nuclei in the control and sham 1 group (Figure 1A and 1B). In contrast, tissue sections in the sham 2 group (recipient of thioacetamide) in comparison with the control and sham 1 groups indicated hepatocyte necrosis, increase in mitosis, cell death, abnormal mitotic inflammation in portal space and large nuclei (Figure 1C). Although tissue samples of the experimental groups 1 and 2 (receiving 100 and 200 mg/kg fish oil omega-3 supplement and thioacetamide) showed no hepatocyte mitosis, apoptosis and necrosis, they had large nuclei (Figures 1D and 1E), whereas the experimental group 3 (recipient of 300 mg/kg fish oil omega-3 supplement and thioacetamide) showed normal liver tissue (Figure 1F).
A, Hepatic tissue of the control group showing normal hepatocytes with protected cytoplasm and marked nuclei; B, hepatic tissue of the sham 1 group showing normal hepatocytes with protected cytoplasm and marked nuclei; C, hepatic tissue of the sham 2 group (receiving thioacetamide) showing hepatocyte necrosis, increase in mitosis, cell death, abnormal mitotic inflammation in portal space and large nuclei; D, hepatic tissue of the experimental group 1 (receiving 100 mg/kg fish oil omega-3 and thioacetamide) showing no hepatocyte mitosis, apoptosis and necrosis, but still have large nuclei; E, hepatic tissue of the experimental group 2 (receiving 200 mg/kg fish oil omega-3 and thioacetamide) showing no hepatocyte mitosis, apoptosis and necrosis, but still have large nuclei; F, hepatic tissue of the experimental group 3 (receiving 300 mg/kg fish oil omega-3 and thioacetamide) showing regeneration of liver tissue to natural state; hepatocytes have normal size nuclei, and mitosis and apoptosis are not seen.
5. Discussion
Liver injury was also determined by biochemical parameters (plasma SGPT, SGOT, ALP, LDH levels). Many useful medicines such as acetaminophen, and some industrial and environmental toxins can cause severe liver damage through functional interference with reactive free radicals. One of these industrial toxins is thioacetamide. This toxin induces hepatic centrilobular necrosis, liver cirrhosis and hepatocellular carcinoma (HCC) [19].
According to the results of this study, the values of albumin, bilirubin, SGPT, SGOT, LDH, GGT in groups treated with thioacetamide significantly increased compared to the control and sham1 group. The average concentration of SGPT in the experimental group which received 100 mg/kg of fish oil omega-3 supplement and thioacetamide showed a significant decrease compared to the group receiving thioacetamide. The mean serum albumin concentration in all experimental groups receiving fish oil omega-3 supplement and thioacetamide significantly decreased compared to the thioacetamide group. The average concentration of billirubin in the experimental groups which received 100 mg/kg of fish oil omega-3 supplement and thioacetamide showed a significant decrease compared to the group receiving thioacetamide. The mean serum GGT in the experimental groups receiving 100, 200 mg/kg of fish oil omega-3 supplement and thioacetamide significantly decreased. This means that the fish oil omega-3 supplement had protective effects on liver cells against damage caused by thioacetamide. The histopathological studies also confirmed these results.
Similarly, Chen et al. showed that DHA has beneficial effects on cholestatic liver disease. The beneficial effects of DHA supplement are related to its strong anti-inflammatory and anti-oxidative effects as well as down-regulation of NF-kB, signaling of the transforming growth factor-beta and Smad protein through functional interference in the activity of extracellular signal regulating kinase (ERK) [20]. More recently, Sherif et al. found that cod liver oil can improve damage induced by sodium nitrite through several mechanisms, including blocking of cell death signs, fibrotic mediators and inflammatory cytokines induced by sodium nitrite [21]. Other evidence showed that DHA supplement and fish oil EPA omega-3 may be the preventive agents in the treatment of liver cirrhosis in mice [22].
According to Li et al. showed that consumption of diet containing fish oil can reduce systemic inflammation and liver damage induced by infection through up-regulation of the peroxisome proliferator-activated receptor gamma-mediated pathway (PPAR) in septic mice [23]. Also, Jangale et al. determined that fish oil and flax seed oil can alleviate inflammation in diabetic mice induced by streptozotocin-nicotinamide [24]. Kim et al. showed that diet containing omega-3 can attenuate Hepatic damage caused by ischemia and tissue Reperfusion via reduction of NF-Kb activity [25]. At the same time, Popescu et al. indicated that omega-3 fatty acid along with diet containing natural calorie and diet with natural lipid has protective effects on nonalcoholic fatty liver disease [26]. Other studies have shown that fish oil diet prevents hepatocyte cancer in B6C3F1 mice [27]. Similarly, omega-3-rich fish oil improves liver damage caused by LPS through the inhibition of TLR4 signaling pathway and NOD [28].
De Meijer et al. showed that emulsion based on fish oil prevents parenteral nutrition-associated liver disease [29]. Also, Khan et al. demonstrated that fish and flax seed oil can protect against apoptosis, tissue damage and hepatotoxicity induced by nitric oxide; it can reduce lipid peroxidation and improve body’s antioxidant system [30]. Other research showed that the cod liver oil improves hepatic damage induced by sodium nitrite via oxidative stress alleviation, and blocking of MCP-1 and mitochondrial functional response as well as reducing DNA fragmentation [31]. It was also found that due to the presence of the antioxidant compounds, omega-3, lipid emulsion based on fish oil prevents liver diseases associated with intestinal failure [32], since omega-3 fatty acid improves the hepatic inflammatory responses by suppressing inflammatory cytokine production in hepatocytes. In addition, EPA reduces levels of TNF-a and IL-6 in the hepatocytes [33], and DHA improves hepatic injuries induced by valproate through reforming of oxidative stresses and inflammation without having any effect on plasma level of valproate [34]. Moreover, it has been shown that addition of fish oil supplement to parental diet reforms the increased levels of hepatic enzymes resulted from hepatic mal-function related to parental nutrition [35]. Studies have shown that 10% fish oil and 1 g% artichoke leaf can restore hepatocellular carcinoma in rats [36]. Lee et al. determined that omega-3 fatty acid can repair hepatocellular damage caused by obstruction of the bile ducts [37]; it was also shown that fish oil along with allopurinol and verapamil improve hepatic injuries resulting from ischemia by a significant reduction in oxidative stress and hepatic enzymes [38]. In a study by Mardones et al., it was demonstrated that combination of thyroid hormone and fish oil protocol prevents liver damage resulted from tissue injury and ischemia [39]. Furthermore, omega-3 fatty acid prevents acute liver defects and stimulates liver regeneration following 90% hepatectomy in rats [40]. Chiang et al. showed that fish oil can stimulate anti cell proliferation effect of 1- alpha 25-dihydroxy vitamin D3 on hepatic cancer cells [41]; EPA can also improve hepatic toxicity, oxidative stress and inflammation induced by valproate [42]. Omega-3 fatty acids down regulate the TNF-a response to lipopolysaccharide insult, as would be seen in sepsis and this may have a direct role in hepatoprotection [43].
In general the results of this study are in line with the results of other studies. It seems that the oral administration of fish oil omega-3 supplement has protective effect on thioacetamide induced liver toxicity by neutralizing free radicals, stimulating the activity of antioxidant enzymes, and reducing the production of inflammatory cytokinin. As no similar study on the protective effects of fish oil omega-3 supplement on hepatic enzymes and histological changes could be found, it was not possible do a comparative study in this respect. Anyhow, more studies should be conducted to examine the hepatic antioxidant enzymes and molecular changes inducing apoptosis so that the effects of fish oil omega-3 supplement on healing liver toxicity can be determined with higher certainty.
In general, the results of present study showed that fish oil omega-3 supplement in rat model with hepatic mal-function can cause desirable improvements. Thus, if supported by more experiments, it is possible to add fish oil omega-3 supplement to the diet of patients with liver mal-function.