1. Background
Bones are known as motional and protective organs and are capable of rearrangement [1]. During the lifetime, young bones are replaced by old ones. This process is done by bone resorption and formation through osteoclast cells on the one hand and the formation of new bone tissue by osteoblast cells on the other, and this cycle continues throughout life [2]. Bone turnover is determined by biochemical markers of bone resorption and formation; increases in biochemical markers of bone resorption such as C-terminal telopeptides of collagen type I (CTX), hydroxyproline, pridinolin, and reduction in bone formation markers such as osteocalcin and alkaline phosphatase are associated with bone degradation and osteoporosis [3]. Several factors such as clinical disorders hyperthyroidism and increased serum cortisol, gastrointestinal disorders, genetics, age, inactivity, or lack of physical activity affect this and somehow serum or plasma levels of the so-called markers of bone resorption and formation; they are often associated with reduced bone mineral density and osteoporosis [4]. Osteoporosis, known as the silent disease, is the most common metabolic bone disease [4].
However, some studies have reported chronic diseases such as asthma and the drugs to treat these diseases as factors effective in reducing osteoporosis [5]. In addition, patients with asthma and chronic obstructive pulmonary, especially those who are treated with corticosteroids for a long time, are at the risk of osteoporosis [5]. Results suggest that the risk of vertebral fractures and non-vertebral fractures in patients with asthma is 2.6 and 1.4, respectively, compared to healthy participants [6]. Patients with asthma who use oral or inhaled corticosteroids have the lowest levels of bone mineral density and double risk of osteoporosis compared to those who have no history of taking corticosteroids [6]. Almost 55 percent of changes in density, strength, and osteoporosis of asthmatic patients are based on the consumption of corticosteroids [7].
Considering markers of bone formation, osteocalcin is a special marker of osteoblasts’ function, major part of which is placed in the bone extracellular matrix after the synthesis and a little part enters the blood flow [3]. Osteocalcin is the major non-collagenous protein in bone matrix produced by osteoblasts and dentin cells [8]. Clinical studies have revealed that although inhaled corticosteroids are considered the most effective drug therapy in the treatment and prevention of asthma severity, high doses of inhaled corticosteroids, in addition to increasing bone resorption, stop bone formation by inhibiting and reducing osteoblast cell proliferation [9]. Thus, their use is associated with decreased levels of osteocalcin and alkaline phosphatase in patients with asthma [9]. It is specified that the use of corticosteroids in asthmatic patients reduces osteocalcin as a formation marker [10]. An inverse relationship has been reported between osteocalcin levels and the use of corticosteroids in asthmatic patients [10]. Alkaline phosphatase is the marker of osteoblast cell activity and bone formation [11]. In the bone, osteoblasts are also a great source of alkaline phosphatase, and its amount in the cell indicates bone formation ability of osteoblasts [12]. Reduced levels of alkaline phosphatase are associated with bone destruction or reduction of bone formation [11]. Injection of corticosteroids to asthmatic patients is associated with the inhibition of osteoblast activity and reduction of alkaline phosphatase levels [13].
Despite the above evidences, given the fact that the stop of taking corticosteroids by these patients has considerable complications, providing other appropriate strategies in order to prevent or delay the phenomenon of osteoporosis is one of the main objectives of health professionals. Hence, physical activity is an important determinant of bone mass, and its role has been demonstrated in the health of skeletal tissue and increase of bone mass [14]. The findings of a recent study showed that one-session aerobic exercise leads to a significant increase in osteocalcin [15]. In a study, four months of strength training significantly increased bone formation markers and decreased bone resorption markers without any changes in bone mineral density [16]. In the study of Bagheri et al. (2012), one-period aerobic exercise including jogging and aerobics for 40 minutes with 60 to 70 percent of maximum heart rate led to a significant increase in alkaline phosphatase [17]. Despite the so-called evidences, in a recent study by Akgu et al. (2015), 8-week swimming training did not lead to changes in the levels of alkaline phosphatase [18]. Some other studies have noted unchanged levels of osteocalcin and other indices of bone formation following exercise activities in adult men and women [19].
Despite limited and contradictory results on other healthy or sick populations, the role of exercise on bone formation parameters, especially osteocalcin and alkaline phosphatase in patients with asthma, has been less paid attention to. Hence, the present study aimed to determine the effect of 3 months of aerobic exercise on the levels of serum osteocalcin on men with mild to moderate asthma.
2. Methods
Subjects: Subjects of this semi-experimental study were twenty four non-trained adult males with mild to moderate asthma treated with inhaled corticosteroids that participated by accessible sampling. The study protocol was approved by research council and ethics committee of Islamic Azad University, Iran (ethic code: 856). The subjects matched for age (38 ± 6 years) and BMI (31.5 ± 3 kg/m2) and assigned to exercise (aerobic training, 3 months, 3 times/weekly, n = 12) and control (no-training, n = 12) groups by simple randomization. Asthma diagnosis and its severity were determined by Forced expiratory volume in one second/Forced vital capacity (FEV1/FVC) and other respiratory volumes as well evaluation of clinical protests by specialist. Each participant received written and verbal explanations about the nature of the study before signing an informed consent form.
Inclusion or Exclusion criteria: A detailed history and physical examination of each subject was carried out. Inclusion criteria for study group were determined as existing asthma for at least three years. All subjects were non-smokers and had not participated in regular exercise/diet programs for the preceding 6 months. Those that were unable to avoid taking drugs for 12 hours before blood sampling were also barred from participating in the study. Presence of previous coronary cardiac disease, chronic airway disease, and impaired hepatic dysfunction, diabetic and presence of any acute disease were determined as exclusion criteria.
Anthropometric measures: Each subject’s anthropometrical markers were measured. Weight and height were measured in the morning, in fasting condition, standing, wearing light clothing and no shoes. Body mass index (BMI) was calculated by dividing weight (kg) by height in meters squared (m2). Waist circumference and hip circumference were measured in the most condensed part using a non-elastic cloth meter. Waist circumference was measured with a non-elastic tape at a point midway between the lower border of the rib cage and the iliac crest at the end of normal expiration. Hip girth was measured at the level of the greatest protrusion of the gluteal muscles with underwear. Visceral fat and body fat (%) was determined using body composition monitor (OMRON, Finland). Each of these measurements was conducted three times and the average was reported.
Exercise program: Based on what mentioned above, this study aimed to determine the effect of 3 months aerobic training on osteocalcin and ALP in asthma patients with inhaled corticosteroids. Intervention lasted a 3 months aerobic training program, three sessions per week, consisting of a warm-up then a 45 - 60-min treadmill exercise at a work intensity of 55% - 75% of max heart rate followed by a cooling-down period [20, Modified]. Main exercise in each session was running at mentioned intensity with no slope. The heart rate, used to calculate the intensity of exercise, was determined by counting heart beats by polar telemetry. The exercise intensity at first week was 55% of max heart rate that graduate increased at lasted sessions of exercise program. Control subjects were instructed to maintain their habitual activities. All participants were instructed to maintain their usual diet throughout the duration of the study.
Blood sampling and analyses: Blood samples were collected before and 48 hours after lasted exercise session with regard to measure serum osteocalcin and ALP of 2 groups. As, a venous blood sample was collected from all the subjects who came after a 10 - 12 hours overnight fast between the hours of 8 to 9 a.m. Subjects were asked to avoid doing any heavy physical activity for 48 hours before blood sampling. After sampling in ETDA- or serum-tubes, blood was immediately chilled on ice, centrifuged and aliquots stored at -80°C until biochemical analyses were performed. Serum used to measuring Osteocalcin by ELISA method (Enzyme-linked Immunosorbent Assay for quantitative detection of human Osteocalcin, Biovendor, Austria). The inter and intra-assay coefficients of variance and sensitivity of osteocalcin were 1.3 and 5.1% and 0.5 ng/mL respectively. ALP was measured by photometric method (Pars Azmoon-Tehran, Iran) by Autoanalyser (RA-100, Canada). The intra and inter-assay coefficients of variance and sensitivity of osteocalcin were 1.06 and 0.85% and 3 U/L respectively.
Data analysis: All statistical analyses were performed SPSS software (Version 15.0, SPSS Inc., IL, USA). Normal distribution of data was analyzed by the Kolmogorov-Smirnov normality test. Independent sample T-test was used to compare the serum levels of all variables between two groups at baseline. Paired t test was used to determine the mean differences between pre and post-training values of each variable. P < 0.05 was considered statistically significant.
3. Results
Baseline (pre-training) and post training of physical characteristics of the subjects are shown in Table 1. There were no statistically significant differences with regard to the anthropometrical markers between 2 groups at baseline (P > 0.05). Aerobic intervention resulted in significant decrease in body weight, BMI and the other anthropometrical markers in exercise group (P < 0.05) but not in control subjects (P > 0.05) (Table 1).
| Variables | Exercise Group | Control Group | ||||
|---|---|---|---|---|---|---|
| Pre-Training | Post-Training | Sig | Pre-Training | Post-Training | Sig | |
| Age, y | 38.3 ± 5.71 | 38.3 ± 5.71 | - | 39.8 ± 4.35 | 39.8 ± 4.35 | - |
| Height, cm | 174 ± 2.22 | 174 ± 2.22 | - | 173 ± 3.05 | 173 ± 3.05 | - |
| Weight, kg | 94.7 ± 11.7 | 90.9 ± 13.3 | 0.019 | 94.9 ± 8.93 | 93.8 ± 8.44 | 0.242 |
| WC, cm | 106 ± 11.27 | 103 ± 11.6 | 0.009 | 106 ± 10.96 | 105.8 ± 10.8 | 0.166 |
| Hc, cm | 107 ± 8.74 | 104.6 ± 9.14 | 0.012 | 104 ± 11.16 | 103.7 ± 10.2 | 0.411 |
| BMI, kg/m2 | 31.5 ± 3.49 | 30.4 ± 3.97 | 0.023 | 31.7 ± 2.80 | 31.3 ± 2.60 | 0.223 |
| Visceral Fat | 13.7 ± 3.73 | 12 ± 4 | 0.011 | 13.2 ± 1.85 | 12.17 ± 3.46 | 0.326 |
Abbreviations: BMI, body mass index; Hc, hip circumference; WC, waist circumference.
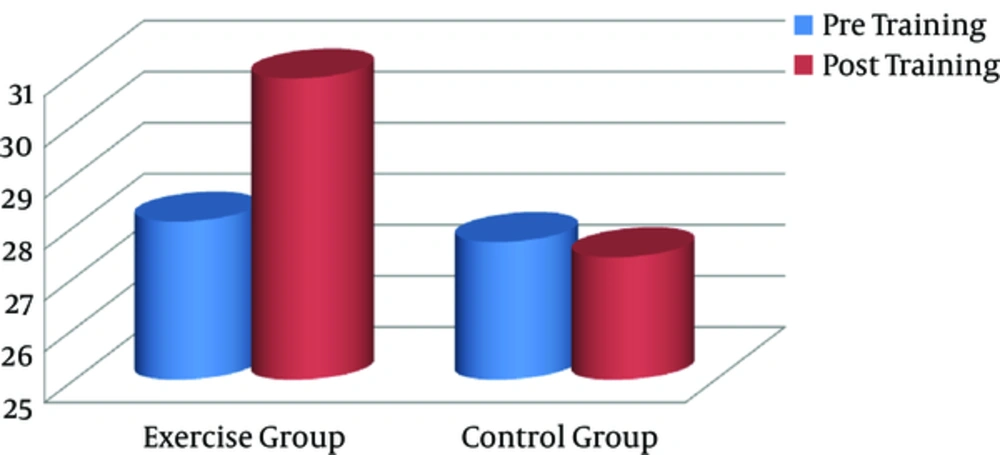
No significant change was observed in serum osteocalcin between exercise and control subjects at baseline (P = 0.87). Exercise group obtained significant increase in serum osteocalcin as a bone formation marker when compared with baseline (P = 0.011). No difference was observed between pre and post-training of osteocalcin in control group (Figure 1).
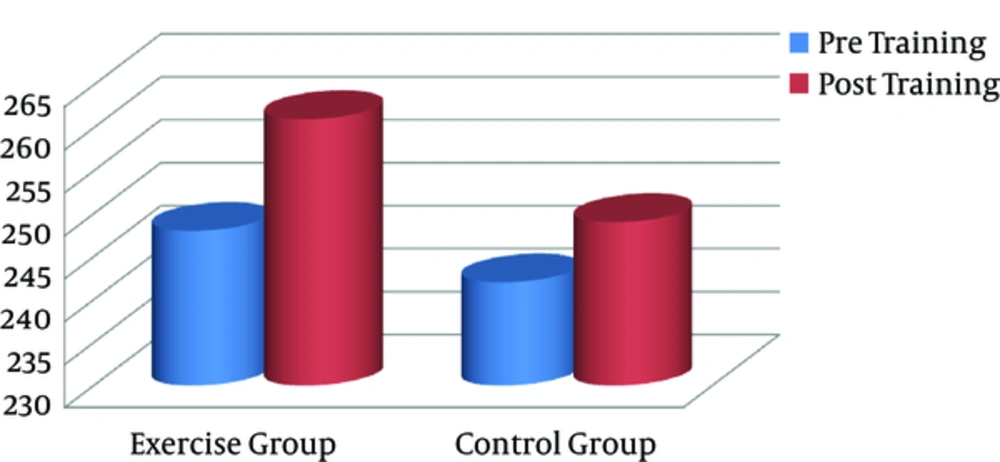
At baseline, there was no significant difference in ALP level between exercise and control subjects. Data by paired T test showed that aerobic intervention was not associated with significant change in ALP in exercise group. On the other hand, no significant change was observed in ALP activity by aerobic training in exercise group. No difference was observed between pre and post-training of ALP activity in control group (Figure 2).
4. Discussion
Despite clear evidences on the potential impact of exercise training on the levels of inflammatory markers in patients with asthma and other chronic diseases, response or compatibility markers of bone formation and destruction to short or long-term training programs have been studied less. On the other hand, the findings of this study refer, somehow, to the beneficial effects of aerobic exercises on the levels of serum osteocalcin, as one of the most important indicators of bone formation in these patients. So that, three months aerobic training consist of three times a week at 55% - 75% HRmax led to a significant increase in osteocalcin levels, as an indicator of bone formation in patients with mild to moderate asthma. However, levels of alkaline phosphatase, as another marker of bone formation in response to aerobic intervention, did not change significantly. Although the response of osteocalcin or other determining markers of bone mineral density or formation to exercise trainings has been less studied in asthmatic patients, their response to a variety of training programs has been reported frequently in some other healthy or sick populations; the findings are, of course, more or less contradictory and heterogeneous. In a recent study by Qadir et al. (2014), 3 months of moderate-intensity aerobic exercise followed three sessions per week, improved markers of bone formation such as alkaline phosphatase, osteocalcin, calcium, and bone mineral density in 30- to 60-year old healthy men and women with normal weight [20].
In another study by Sun et al. (2015), a period of 60-day regular exercise increased bone mass, the number of bands of connective tissue, thickness of connective tissue, bone mineral density, and mechanical power in Wistar rats [21]. In contrast to these findings, in the study of AkguL et al. (2015), a period of swimming training did not lead to a change in bone mineral density, osteocalcin, and alkaline phosphatase in 10- to 22-year-old swimmers [18]. In addition, in the study of Moazami et al. (2013), six months of aerobic exercise did not significantly change parathyroid hormone levels and alkaline phosphatase in inactive obese women [11]. However, in the findings of Pamlas et al. (2006), despite significant increases in bone formation, markers such as alkaline phosphatase increased after 6 weeks of aerobic training in inactive men and women, but bone resorption markers remained unchanged [22]. Despite the inconsistency in the above findings and given the potential impact of long-term use of corticosteroids on bone metabolism, it is concluded that the increase of serum osteocalcin levels in the current study is rooted in the inhibitory effect of aerobic exercise on the role of inhaled corticosteroids in the process of bone formation in patients with asthma. This is due to the fact that inhaled corticosteroids, though, the most effective and accessible pharmacological intervention, have been introduced to control asthma, but their entrance from the lungs into the blood flow has some non-negligible side effects [23].
Inhaled glucocorticoids are widely used to treat and inhibit the severity of respiratory disorders in diseases such as chronic obstructive pulmonary. It has been found that the use of inhaled glucocorticoids delays reduction of lung function in patients with respiratory diseases, although long-term use of them has been emphasized [24]. On the other hand, long-term use of inhaled glucocorticoids, along with high doses of corticosteroids, is associated with osteoporosis or pneumonia [5]. Glucocorticoids penetrate into the cytoplasm through the cell membrane and bind to their receptors in the cytoplasm [25]. Epithelial cells, the main location of asthma symptoms, are the most important target locations of inhaled corticosteroids. Transcription of several inflammatory genes in epithelial cells inhibits the respiratory tracts and leads to a reduction in the inflammation of the lining of the respiratory tracts [23], which increases transcription of lipocalin 1 genes, adrenergic receptors, leukocyte inhibitor protein, anti-inflammatory cytokines such as interleukin 10, interleukin 12, and interleukin 1 receptor antagonists, and inhibition of MAP kinase-dependent pathways in the respiratory tracts. It also reduces the transcription of inflammatory cytokines such as interleukin 2 to 6, interleukin 11, 15, and TNF-α, chemokines transcription such as interleukin 8 and etakcin, inflammatory peptides such as endothelin 1, and decreases transcription of adhesion molecules such as ICAM-1 and VCAM-1 in the respiratory tracts. These effects confirm the efficacy of inhaled glucocorticoids on the inhibition of asthma symptoms [23].
However, high-dose use has adverse systemic effects including bone diseases such as rickets, osteoporosis, and bone necrosis. Corticoids have damaging effects on the function and survival of osteoblasts and osteocytes, and on the maintenance or prolonging of the lifespan of osteoclasts that are associated with metabolic bone diseases. Osteoporosis caused by corticosteroids is associated with some abnormalities such as fracture of the vertebrae of the spine and femoral neck [26]. Glucocorticoids reduce the process of osteoblastogenesis, increase osteoblasts’ cell death, and reduce their ability of bone formation [27]. Taking glucocorticoids also changes the potential of bone marrow derived mesenchymal stem cells, called Adipogenesis Differentiation, and is associated with osteoblastogenesis [28]. Jan et al. (2000) found that one-time injection of corticosteroid to36 asthmatic kids immediately suppressed osteoblast cell activity and decreased the levels of osteocalcin [10]. Also, an increase in the production of steroid-induced reactive oxygen species leads to cell death of osteoblasts [29].
Studies on mouse samples have shown that the activity of glucocorticoid receptors in osteoblasts reduces the bone mass, the thickness of the bone connective tissue bands, the number of osteoblasts, and colony forming units [30]. Inhibiting the activity of cytokines derived from osteoblasts by steroids such as interleukin-11 also damages the differentiation of osteoblasts [30]. These factors all decrease levels of osteocalcin via long-term use of corticosteroids. On the other hand, based on the findings of this study, it is concluded that increased osteocalcin serum levels through regular aerobic exercises bring about the beneficial effects of exercise even in the presence of corticosteroids. Improvement of osteocalcin levels or other markers of bone formation in response to exercise trainings has also been reported by some other studies [20]. Some studies have indicated that aerobic exercise alone is not enough to maintain or improve the function of bone formation markers. It is indicated that weight-bearing exercises are necessary for the prevention of osteoporosis in at-risk individuals, while aerobic exercises such as running on a treadmill are not enough to maintain bone mass [31]. However, in a two-year prospective study, a training program was designed with the aim of determining bone mineral density in a group of women who were in their early post-menopause period; 24 months aerobic exercise was performed to maintain bone mass in the upper end of the femur and spine. Intense aerobic exercise increased growth hormone secretion and the mechanical strength and stimulated bone formation [32].
The effects of some other exercises on the markers of bone formation, such as swimming that does not endure body weight, are somewhat controversial. In a recent study, a long-term swimming training course did not lead to a change in bone mineral density, osteocalcin, and alkaline phosphatase in 10- to 22-year-old swimmers [23]. However, researchers have noted that different pressures and hits when swimming can cause another type of pressure or resistance on the bone. Falcai et al. (2015) found that all three training programs in swimming, jumping, and vibration significantly increased bone mass, bone strength, bone formation, and the serum levels of markers of bone formation in Wistar rats [33]. Accordingly, prescribed exercises should be designed depending on the level of tolerance of each patient, especially those at risk of osteoporosis such as those who use inhaled or systemic corticosteroids [34].
In the end, it should be noted that small number of patients is a one of the limitations of this study. In addition, lack measuring CTX as bone resorption marker is the weak points and main limitation of your study. Because, CTX measuring as one of main marker of bone turnover give us important information about the effects of exercise training on bone metabolism.
Conclusion: Despite the efficacy of inhaled corticosteroids on respiratory function of patients with asthma, clinical studies have supported their adverse effects on bone metabolism. However, some external stimuli such as performing regular exercise trainings are associated with improved markers of bone formation in these patients; 3 months of aerobic exercise significantly increased osteocalcin levels as one of the markers of bone formation in patients with asthma. Hence, based on the findings of this study, implementation of regular moderate-intensity aerobic exercise trainings is suggested in order to maintain or improve bone metabolism in those asthmatic patients who use inhaled corticosteroids.