1. Background
Fragrances are widely used and they contain anthropogenic nitrobenzene derivatives [1]. Some of the most commercially used compounds in fragrances include musk moskene (MM) (1, 1, 3, 3, 5-pentamethyl-4, 6-dinitro-2H-indene), musk tibetene (MT) (1-tert-butyl-3, 4, 5-trimethyl-2, 6-dinitrobenzene), musk ambrette (MA) (2, 6-dinitro-3-methoxy-4-tert-butyltoluene), musk ketone (MK) (4-tert-butyl-2, 6- dimethyl-3, 5-dinitroacetophenone) and musk xylene (MX) (1-tert-butyl-5-dimethyl-2, 4, 6-trinitrobenzene) [1]. MM and MT have been banned in the United States [1] and MA has been associated with various adverse effects in rats such as hind limb weaknesses and pathologic effects on neural tissue [2]. MK and MX however are still being used promiscuously in perfumes and as additives in household cleaning products and other fragrant non-cosmetic products [3]. The European Commission Joint Research Centre Institute for Health and Consumer Protection in 2008 classified MK and MX as environmentally ubiquitous, very persistent and very bio-accumulative compounds which are not easily bio-degradable [4].
Creatine kinase (CK), also referred to as creatine phosphokinase (CPK) or phospho-creatine kinase is an enzyme that catalyses the conversion of creatine (utilising adenosine triphosphate (ATP)) to phosphocreatine (PCr) and adenosine diphosphate (ADP). The reaction allows for reversible ATP generation [5].
In tissues and cells that consume ATP rapidly, especially brain and striated muscles, PCr serves as an energy reservoir for the rapid buffering and regeneration of ATP in situ, and for intracellular energy transport [5, 6]. CK exists in three cytosolic and two mitochondria isoforms. Isoform patterns occur differently in tissues. In the cytosol are CKB (brain type), CKM (muscle type) and CKMB (mixed type); and in the mitochondria are ubiquitous mitochondrial CK (uMtCK) and sarcomeric MtCK (Ckmt2) [6]. CKB (brain) is expressed in all tissues at low levels and has little known clinical significance. Skeletal muscle expresses CKM (over 95%) and low levels of CKMB (1%). Cardiac muscle expresses CKM at 70% and CKMB at 25% - 30% [5, 6]. Clinically, CK is assayed as a screening test for suspected muscle disease including myocardial infarction, rhabdomyolysis, muscular atrophy and acute renal failure. It is a useful diagnostic test because its levels are not falsely raised by haemolysis, and is readily released by cellular injury because it is unbound in the cytosol [5].
In Nigeria and around the world, even though the precise formulae of commercial perfumes are kept as trade secrets, the effects of these fragrance products on myocardial function need to be properly elucidated. This is because consumers of fragrance products are habitually and intentionally exposed to the complex mixtures found in perfume products [1, 3]. A thorough literature search did not provide studies investigating the effects of fragrance products on cardiac muscle stress.
2. Objectives
This study was designed therefore to determine the effects of exposure to two locally made perfumes; (“Miyaki” and “Cool River” designated F1 and F2 respectively) on the gene expression of Ckm and Ckmt2 and the activity levels of total CK in the hearts of Wistar rats.
3. Materials and Methods
3.1. Animals, Grouping and Treatment
In this experimental study, the maintenance and care of experimental animals complies with national institutes of health guidelines for the humane use of laboratory animals and approval was obtained from the College of Health Sciences research committee, university of Ilorin, Nigeria. Eighteen healthy male Wistar rats (average weight 148 g) were obtained from and kept at the animal holdings unit of the department of anatomy, university of Ilorin in well ventilated cages under standard photoperiodic conditions and given rat pellets (Bendels Feeds, Yoruba road, Ilorin) and water ad libitum. Rats were divided into three groups labeled A, B and C. Group A is the control group while B and C were the experimental groups. All groups consist of 6 rats each. During each day of administration, 5 mL of each perfume (F1 for group B and F2 for group C and 5 mL of distilled water for group A) was injected into balls of cotton wool. The cotton wool was placed in a perforated container so as to prevent the rats from eating the soaked cotton wool. The rats were exposed to the fragrance of perfumes for 6 hours daily after which they were returned to their respective cages. The administration was conducted for 77 days. After exposure, hearts were excised, rinsed in ice-cold isotonic saline, blotted with filter paper, and homogenized for CK activity. Portions of the hearts were also removed and fixed in 10% formalin solution for paraffin embedding.
3.2. Creatine Kinase Activity Assay
According to the CK Activity Colorimetric Assay kit, CK converts creatine into phosphocreatine and ADP. The generated phoshocreatine and ADP reacts with CK enzyme mix to form an intermediate, which reduces a colorless probe to a colored product with strong absorbance at 450 nm. The manufacturer’s protocol was followed for the assay, briefly; 10 mg of heart tissue was rapidly homogenized with 100 μL ice cold assay buffer for 10 minutes on ice. The homogenate was centrifuged at 12000 rpm for 5 minutes. The supernatant was collected and 10 μL of the sample was added per well of a 96-well Falcon tissue culture plate. The final volume was adjusted to 50 μL with assay buffer. For the positive control, 10 μL of positive control was added into appropriate wells and final volume adjusted to 50 μL with assay buffer. Fifty (50) μL of the reaction mix was added to each well containing the standard, positive control and samples, mixed well, incubated for 30 minutes at 37ºC and the optical density was measured in a microplate reader (Anthos 2010 model 17-550 Austria) at a wavelength of 450 nm.
3.3. Quantitative Real-Time PCR
Extraction of total RNA from formalin fixed, paraffin embedded tissue was performed as described by Ma et al. [7]. The total RNA was precipitated by mixing with 0.6 mL isopropyl alcohol, and placed at -20ºC for 1 hour and then centrifuged at 12000 × g for 10 minutes at 4ºC. RNA pellet was washed with 100% ethanol, briefly air-dry and dissolved in RNase-free water. RNA concentration and purity were determined by using the Nanodrop-1000 spectrophotometer. RNA integrity was checked by gel electrophoresis. According to the manufacturer’s instructions, genomic DNA was removed from total RNA using the DNase I, RNase-free kit. The DNase I treated RNA was again cleaned with the Gene JET RNA Purification kit, re quantified and stored at -80ºC until used. According to the manufacturer’s instructions, cDNA was synthesized using the MultiScribe™ Reverse Transcriptase from 500 ng RNA. The reverse transcriptase reaction was carried out in a GeneAmp® PCR System 9600 Thermal Cycler for 10 minutes at 25ºC, 120 minutes at 37ºC and then the enzyme was deactivated for 5 minutes at 85ºC. The cDNA aliquots were then utilized in qPCR reactions for Ckm, with Sdha, Actb and B2m used as the endogenous reference genes. PCR reactions were amplified for 40 cycles prior to which the AmpliTaq Gold® DNA polymerase was activated for 10 minutes at 95ºC. Each cycle consisted of a denaturing step for 15 second at 95ºC and annealing/extension step for 1 minutes at 60ºC. PCR amplification was performed in a final volume of 20 μL using the Power SYBR® Green PCR Master Mix with the ABI 7500 real-time PCR machine. Primer sequences and PCR product sizes are indicated in Table 1. Primers were designed with NCBI/Primer3 and BLAST.
| Gene and Sequences (5'-3' Direction) | NCBI Accession No. | Product Size, bp |
|---|---|---|
| Ckm | NM_012530.2 | 123 |
| F: CAC CCC TTC ATG TGG AAC GA | ||
| R: CTC AAA CTT GGG GTG CTT GC | ||
| Ckmt2 | NM_001127652.1 | 79 |
| F: CCC CCA AGT GCA GAC TAT CC | ||
| R: TGG CAT AGA TGG TTG GGG TG | ||
| Sdha | NM_130428.1 | 102 |
| F: CCC ATT CCA GTC CTT CCC AC | ||
| R: CAC AAT CTG ATC CTG GCC GT | ||
| Actb | NM_031144.3 | 134 |
| F: CTG TGT GGA TTG GTG GCT CT | ||
| R: AGC TCA GTA ACA GTC CGC CT | ||
| B2m | NM_012512.2 | 170 |
| F: CAC ACG CAG TCT GAA AAC CC | ||
| R: AAA GGA GCT TTG GGG ACA CA |
To confirm the absence of nonspecific amplification, PCR products were separated on 3% agarose gels, stained with ethidium bromide and images acquired with the BioRad Gel Doc® XR (Model 170-8170 Segrate, Milan, Italy). Melt curves were generated for each PCR product using the Applied Biosystems ABI 7500 software. The relative mRNA expression levels of target genes in each sample were calculated using the qbasePLUS software (Biogazelle, Zulte, Belgium).
Statistical Analysis: Statistical analysis was performed using JMP® (Version 10.0 SAS Institute Inc., Cary, NC). Data are reported as mean ± SEM. After verifying the normal distribution and the homogeneity of the variance using an F-test (p<0.05), a one way analysis of variance (where a significance level of P < 0.05 was set) was used to compare the results. All means were then compared using the Tukey-Kramer HSD.
4. Results
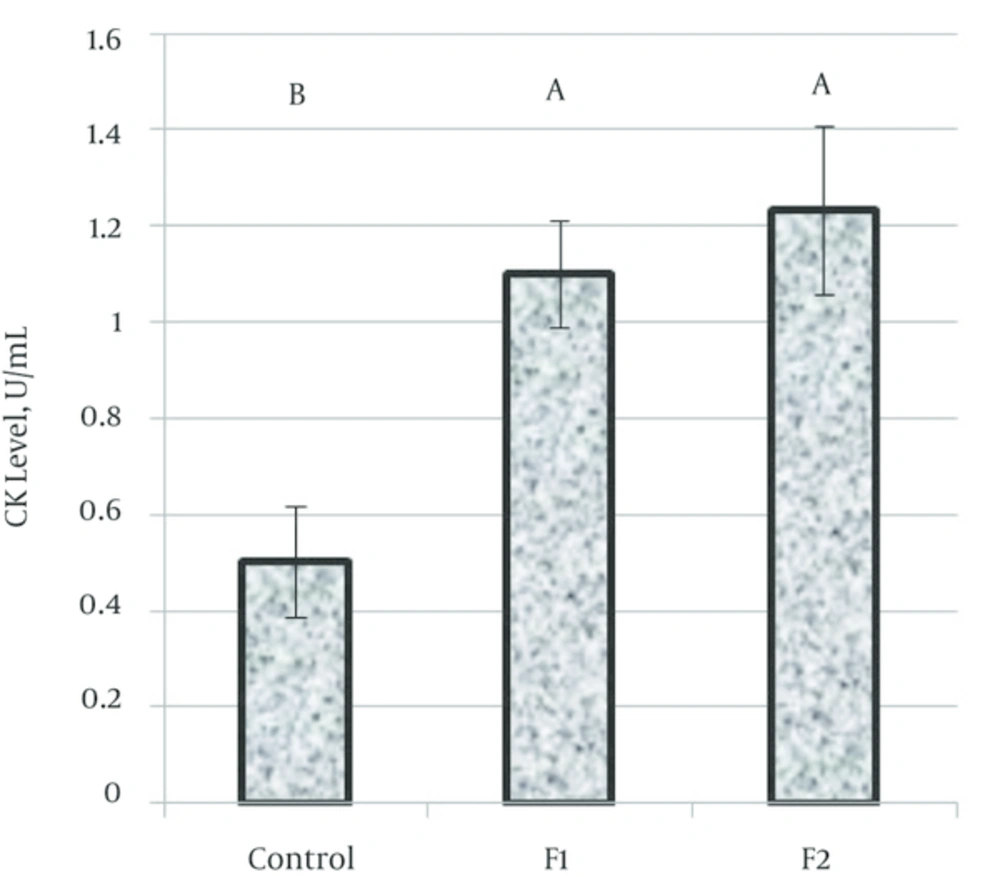
Total CK activity: Levels of creatine kinase (CK); indices of cardiac cellular damage, were assessed and found to be significantly (P = 0.0001) higher in rat groups exposed to both fragrances for 77 days as compared to the control groups (Figure 1).
4.1. Expression of Ckm and Ckmt2 Genes
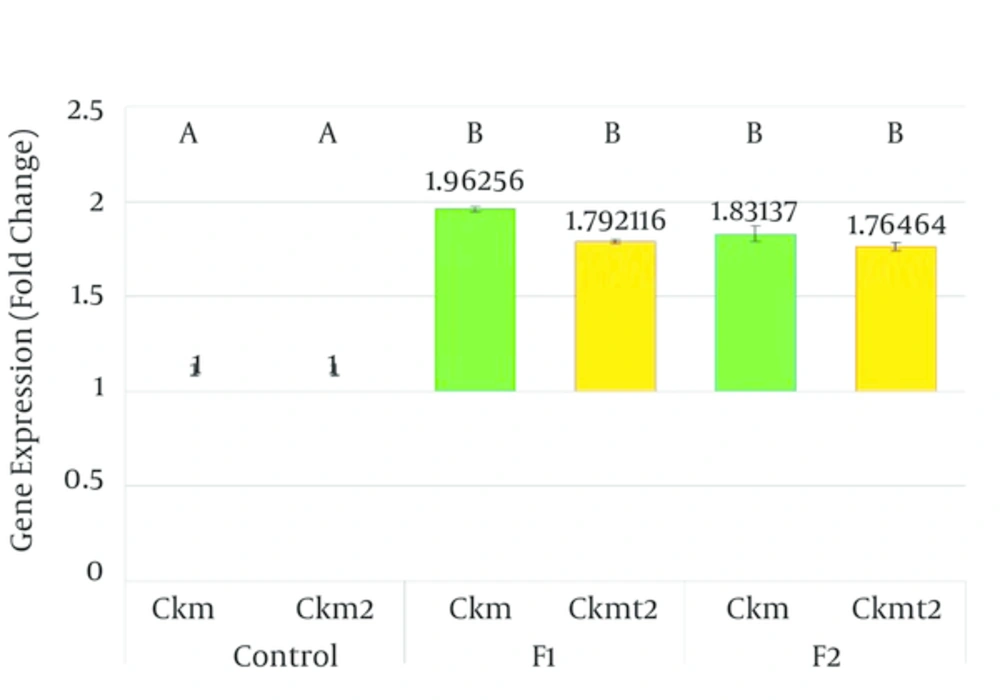
The mRNA expressions of the two creatine kinase isoforms investigated were found to be significantly altered in the groups of animals exposed to both fragrances. Ckm mRNA was significantly (P = 0.0001) up regulated in rat groups exposed to both fragrances (Figure 2). Striated muscle associated sarcomeric mitochondrial CK mRNA was also significantly (P = 0.0001) up regulated in both fragrance exposed groups (Figure 2).
5. Discussion
The increased expression of striated muscle associated creatine phosphokinase and sarcomeric mitochondria Ck genes as well as the increased release of the cardiomyocyte enzyme CK in the hearts of Wistar rats suggests that exposure to these two locally made fragrances contributes to cardiomyocyte stress. Creatine kinase is involved in the maintenance of striated muscle intracellular energy supply through its ATP buffering capabilities [8, 9]. Ck thus play very important roles in tissues requiring fluctuating and enormous energy demands like cardiac muscle and brain, with the mitochondrial isoform of CK (MtCK) required for the energetics of oxidative tissue [9].
The production of reactive oxygen and nitrogen species (ROS, RNS) is central to the pathophysiology of myocardial injury [9, 10] and this is often characterized by oxidative damage, poor energetic state of tissues, calcium overload and increased apoptosis. The impairment of CK isozymes by ROS/RNS leads to the interruption of the CK/phosphocreatine-shuttle and cause impaired cellular energy dynamics [9]. Upregulation of CK isozymes in the rat hearts has been associated with stress by chronic restrain, demonstrating cardiac malfunction, impaired mitochondrial respiration and apoptosis [11].
Consumers of fragrance products are habitually and intentionally exposed to the complex mixtures found in perfume products [1, 3, 12]. The chemical mixtures of fragrance products may contain 100 or more fragrance raw materials (FRMs) at concentrations of 1% or greater. Many previous toxicology studies [13-16] assessed the hazards of exposure to just one to three components out of the numerous compounds contained in the perfume mixtures, whereas actually, humans are exposed to the complex numbers of chemicals contained in fragrance mixtures [12]. One of the objectives of this study therefore was to evaluate the safety of the prolonged use of finished fragrance products via the inhalation route in rats. A thorough literature search did not provide studies investigating the effects of fragrance products on cardiac muscle stress. Many studies however were found demonstrating the developmental, carcinogenic and human endocrine effects of fragrance compounds [12, 14, 16]. Carlsson et al. [13] reported that MK levels of 0.1 - 10 mg/g of food per day reduced the body weight of spawning females as much as 38% and reduced the number of eggs per female per day as much as 95% in zebra fish. South African clawed frogs exposed to 400 μg/L/day of MK, MX or MM in the surrounding water for eleven days demonstrated increasing larvae mortality for each increasing day of exposure [17]. The lipophilicity of nitro musks has raised concerns over their possible endocrine modulating effects. When human MCF-7 breast cancer cells were exposed to MX or MK in surrounding media, there was a significant increase in the proliferation of the human breast cancer cells by 29% for MX and by 97% for MK when compared to the negative control [14]. While various genotoxicity studies (Api and Gudi, Kevekordes et al.) in Escherichia. coli PQ27 cells, human hepatoma, human lymphocytes cell lines and rats indicated that nitro musks were not genotoxic [18-20], it has been suggested that other in vivo processes might be occurring to promote tumorigenesis as shown by the report of Maekawa et al. [21], which found that mice fed with food containing 0.075% or 0.15% MX had three times as many tumors compared to control mice.
In this study, we report that levels of creatine kinase (CK) and the mRNA expressions of two creatine kinase isoforms (Ckm and Ckmt2); important indices of cardiac cellular damage, were assessed and found to be significantly higher in rat groups exposed to two locally made Nigerian perfumes.