1. Background
During the last decade, St. John’s wort (Hypericum perforatum) has gained popularity as an alternative treatment for depression and a natural remedy for reducing inflammation due to its especial chemical composition [1, 2]. H. perforatum is a perennial herb of the Hypericaceae family native to parts of Europe and Asia (middle-east and other parts) and also has spread worldwide, including temperate regions of India, China, Africa, and some parts of the United States and is distinguished by its golden yellow calycles [1]. In addition, when flower buds are crushed, a reddish liquid would be exposed. This reddish liquid contains numerous compounds with biological activity such as phloroglucinols hyperforin, adhyperforin, the naphthodianthrones hypericin and pseudohypericin, the flavonoids hyperoside, quercetin, rutin, quercitrin and isoquercitrin, and the dimeric flavonoids amentoflavone [3]. Oily Hypericum preparations have been used for centuries as a medicinal plant to treat burns, wounds, alcoholism, dermatitis, neuralgia and myalgia beside its antidepressant, antiviral, and antibacterial effects [4-7]. Nowadays, Hypericum is used widely as an antidepressant medication for the treatment of mild to moderate depression [8, 9]. Since, the botanical products of Hypericum are commercially available over-the-counter, some physicians prefer to prescribe them instead of synthetic medicines such as fluoxetine to treat mild to moderate depression.
Different studies have been done on Hypericum anti-depressant effects, but only a few ones have documented its in vivo anti-inflammatory properties following systemic administration. It has been shown that the hydro-alcoholic extract of H. perforatum caused a significant reduction in the production of pro-inflammatory cytokines; IL-17 and IFN-γ as well as the level of IL-6 in NMRI mice immunized with sheep red blood cells [10]. In 1999, Bork et al. showed that H. perforatum extracts inhibit the transcription factor NF-kB [11]. Hypericin as one of the major components of H. perforatum extracts have antioxidant activity and also inhibits IL-1 and IL-12 production by innate immune cells [12, 13]. Also, H. perforatum extract contains other components like phloroglucinol possess antioxidant properties by reducing ROS and NO production and inhibits IL-6 production [14-16].
Inflammatory bowel disease (IBD) is a group of inflammatory conditions of the colon and small intestine [17]. The two major clinical forms of IBD are Crohn’s disease and ulcerative colitis, which both are chronic remittent or progressive inflammatory conditions that may affect the entire gastrointestinal tract or some parts of it. Although, the main etiology of IBD is unknown, the occurrence of IBD is likely to depend on multi-genetic predisposition and immune system responses to microbiome according to available literatures [18]. Basically, interactions between the intestinal microflora and the mucosal immune system and the way environmental factors alter these relationships appear particularly relevant for the development of IBD [19]. Current available treatments do not provide an absolute cure and just reduce the inflammation somehow [20]. These treatments are limited due to their inefficacy, toxicity, drug interaction and side effects, hence, it seems that consuming a natural agent with moderate anti-inflammatory effect may be assuagement [21].
Hypiran is a hydro-alcoholic extract derived from the H. perforatum herb which contains 0.2 mg/ml hypericin. Hypiran is recommended for a variety of ailments such as depression, insomnia, anxiety, headache, menstrual headache and migraine [22]. With regard to anti-inflammatory benefits of H. perforatum, the aim of this study was to peruse the potential effects of Hypiran for the treatment and controlling inflammation in animal models of ulcerative colitis.
2. Methods
2.1. Reagents
Hypiran was purchased from Pursina Pharmacy (Tehran, Iran). Prednisolone was provided by Aburaihan Pharmaceutical Co. (Tehran, Iran). The enzyme-linked immunosorbent assay (ELISA) kits were procured from Qiagen (Hilden, Germany). Total protein assay kit was purchased from Zist chemi Co. (Tehran, Iran). Other reagents were obtained from Sigma-Aldrich (St. Louis, MO).
2.2. Animals
The male Wister albino rats weighing 280 - 300 g were obtained from experimental animal care center of Urmia University. The rats were housed under controlled environmental conditions (25°C and a 12 hours light/dark cycle), and received food and water ad libitum. All procedures in this study were conducted in accordance with the regulations of the ministry of health and medical education of I.R. Iran, approved by the medical ethics committee of the University for Animal Studies.
2.3. Induction of Colitis and Evaluation
Colitis was induced as described previously [23]. In brief, rats were fasted for 36 hours. Afterward, the animals were anesthetized and intra-rectally received 2 mL solution of 4% acetic acid by a rubber cannula (8 cm long). Rats were randomly assigned into the following groups (n = 10): The first group served as normal control rats and received no treatment; the second group, colitis control rats, received only vehicle (0.5 ml of PBS, PO, daily). Hypiran treated group and positive control were consisted of rats with colitis treated with Hypiran) 150 mg/kg, PO, daily) or Prednisolone (4 mg/kg PO, daily), respectively. Therapies were initiated on the day of induction and continued throughout the study until the day 10 when rats were sacrificed.
Body weight, stool consistency and gross bleeding were monitored daily. Disease activity index (DAI) was quantified as the sum of scores of weights loss, stool consistency and blood feces according to the parameters outlined in Table 1. The survival rate of rats was recorded daily during the study. Also, the colon samples were dissected from 10 cm distal colon segments, and macroscopic examinations (hemorrhage and ulcer formation) were conducted on a white screen.
2.4. Colonic Homogenate
The distal colon was weighed and the same amount of tissue was cut, opened and homogenized in 10 volumes of ice-cold physiological saline. The homogenized colon tissue was centrifuged at 10,000 g at 4°C for 10 minutes [24].
2.5. Evaluation of Myeloperoxidase (MPO) Activity in the Colonic Tissues
In brief, 10 µL of homogenized colon tissue were mixed with 80 µL of 0.75 mM H2O2 and 110 µL TMB solution (2.9 mM TMB in 14.5% DMSO plus 150 mM sodium phosphate buffer at pH 5.4). The absorbance was read immediately at 450 nm (reference: 620 nm) on a microplate reader. Then, the sample was incubated at 37°C for 15 minutes. Next, for termination of the reaction, 50 µL of 2 M H2SO4 was added and the absorbance was determined spectrophotometrically at 450 nm. For the assay, 10 µL of horseradish peroxidase (HRP) (2.5 and 25 milliunit/mL HRP) were used. MPO activity was calculated as the difference of the absorbance relating to the HRP standard curve. Data was expressed as milliunits per milliliter (mU/mL) [25].
2.6. Determination of Nitric Oxide Levels in the Colonic Tissues
The level of nitric oxide in the colon was checked by using the Griess method. 50 µL of homogenized colon tissue were combined with 50 µL Griess reagent (0.1% sulphanilamide, 3% phosphoric acid and 0.1% naphthyl ethylenediamine) and then maintained at room temperature for 10 minutes in the dark. Next, the absorbance was recoded at 540 nm on a microplate reader. The nitrite concentration was checked based on the standard curve [26].
2.7. Malondialdehyde (MDA) Determination in the Colonic Tissues
The level of malondialdehyde in the colonic tissues was assessed similar to the procedure described earlier [27]. In brief, one hundred microliters of colon homogenate were mixed to 2.5 ml reaction buffer (0.37% thiobarbituric acid, 0.25 M HCl and 15% trichloroacetic acid, 1:1:1 ratio) and heated at 95°C for 60 minutes. Afterwards, the mixture was cooled down and centrifuged at 3,500 g for 10 minutes. Finally, the absorbance of supernatant was recoded at 540 nm on a microplate reader. Data were expressed as nM of MDA/mg protein.
2.8. Assessment of Inflammatory Cytokine Levels in Colon
TNF-α, IL-1β and IL-6 levels were determined in colonic homogenate by enzyme-linked immunosorbent assay according to manufacturer’s methods [28].
2.9. Determination of Total Protein Levels in Colon
After preparation of homogenized colon tissue, total protein in the colon was assessed and quantified determined by pyrogallol red-molybdate method according to the manufacturer’s instructions [29].
2.10. Statistical Analysis
When the values were nonparametric (discontinuous ranks related to the severity of the disease), the Kruskal Wallis test followed by Mann Whitney U evaluation with Bonferroni adjustment was applied to compare score differences between the groups. The rest of the data were continuous data, and after confirmation of their normal distribution by Kolmogorov-Smirnov test analysis, these parametric data were assessed, using the one-way ANOVA, plus Dunnett’s post hoc test. Statistical analyses were done by SPSS Windows 23.0 statistical analysis software (SPSS Inc., Chicago, IL, USA). The Kaplan-Meier estimator was applied to report the survival function of lifetime data. Differences in the survival rate were analyzed by log-rank testing. MedCalc Software (version 15.8, MedCalc Software, Mariakerke, Belgium) was used to analyze the Kaplan-Meier estimator. Data were presented as means ± SEM. The P values of less than 0.05 were considered statistically significant.
3. Results
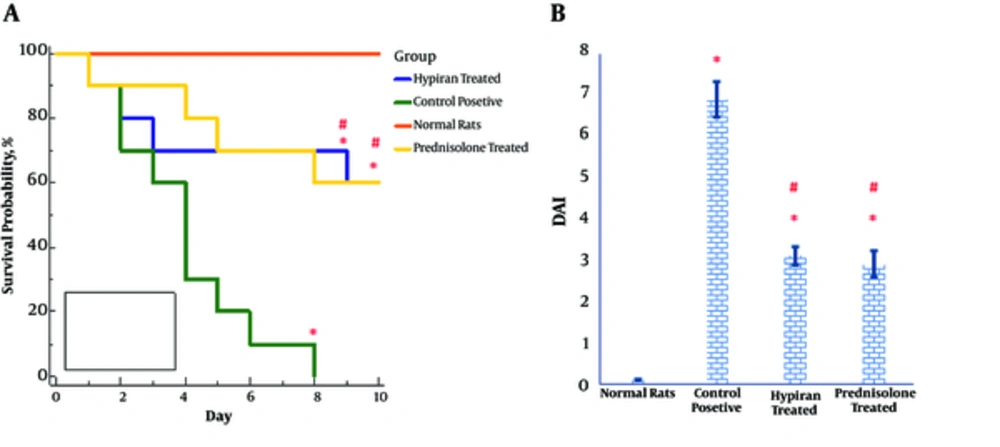
Luminal instillation of acetic acid into the colon of rats resulted in a high DAI score and a high mortality rate. Nevertheless, pharmacotherapy with each of the drugs (Hypiran or Prednisolone) equally led to a reduction in DAI score and a mortality rate (Figure 1A and 1B). As shown in Figure 1A, all control positive rats died within 8 days after luminal instillation of acetic acid, nevertheless, normal rats didn’t have any death in this period. The survival rate over during 8 days of study in the Hypiran or Prednisolone group was 60% (6/10 rats).
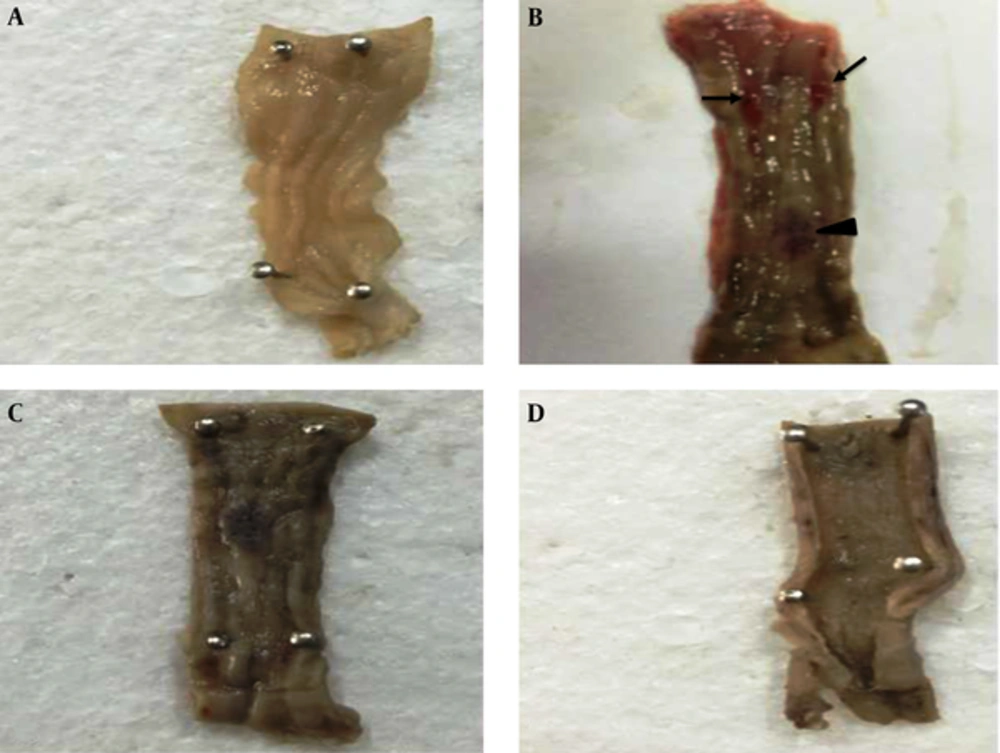
Gross pathology examination also showed that both therapies (Hypiran or Prednisolone) could regress ulceration and hemorrhage in the colonic mucosa (Figure 2). To determine the mechanisms involved in the beneficial effects of each medication, especially Hypiran, other experiments were designed.
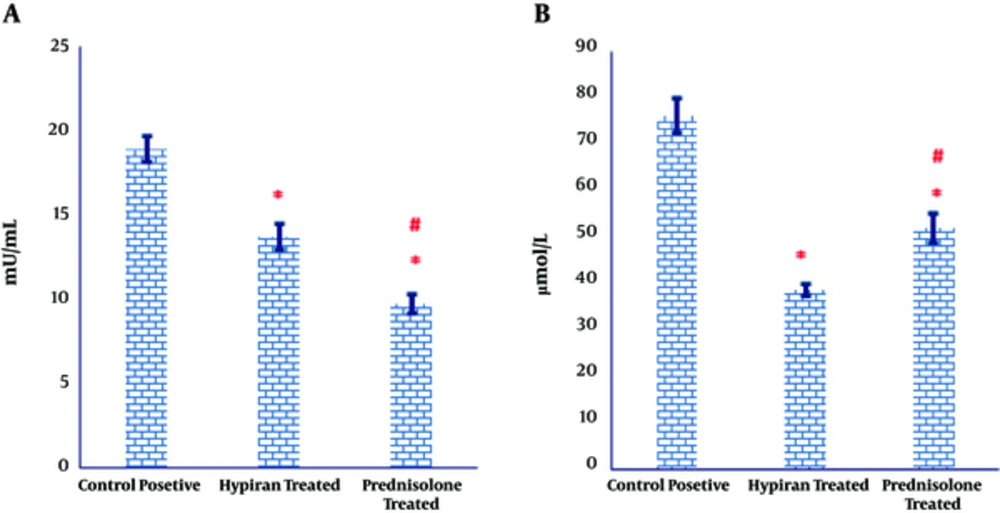
As illustrated in Figure 3A, both medications caused a significant regress in the MPO activity in the colonic tissues of colitis induced rats, compared to the MPO activity in the colonic tissues of control positive group. However, MPO activity showed a more prominent significant decrease in the colonic homogenate of colitis rats received Prednisolone compared to those received Hypiran (Figure 3A).
The levels of myeloperoxidase activity were down-regulated in the gut of Prednisolone traded rats more than Hypiran groups. Conversely, Hypiran decreased the levels of nitric oxide more profound than Prednisolone. (*P < 0.001 versus control positive rats; #P < 0.01 versus Hypiran treated group).
Similarly, the obtained results expressed that the levels of nitric oxide production were significantly suppressed in the colonic tissues of rats with colitis, compared to the nitric oxide level in the colonic tissues of control positive rats (Figure 3B). Nevertheless, this reduction was more eminent in the group treated with Hypiran than the one received Prednisolone (Figure 3B).
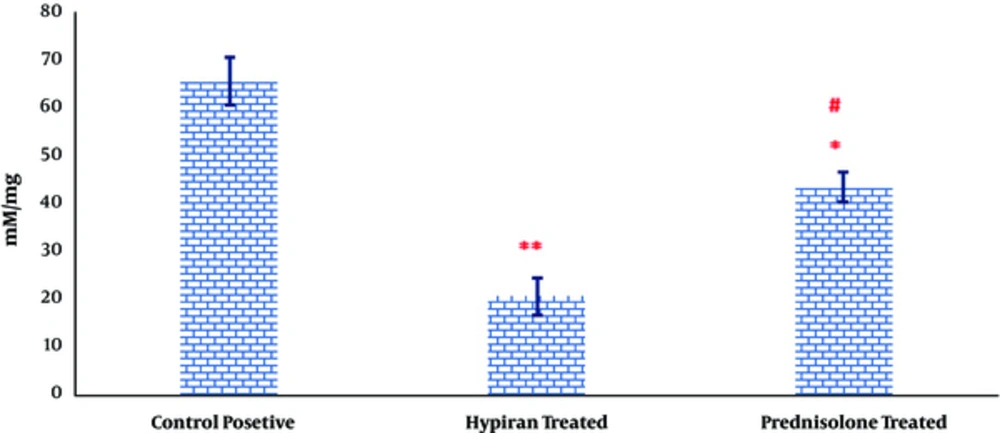
As it can be observed in Figure 4, the levels of MDA were significantly down-regulated in rats with colitis received Hypiran or Prednisolone compared to the colitis group without any treatment. However, this reduction was more prominent in the Hypiran group than Prednisolone group.
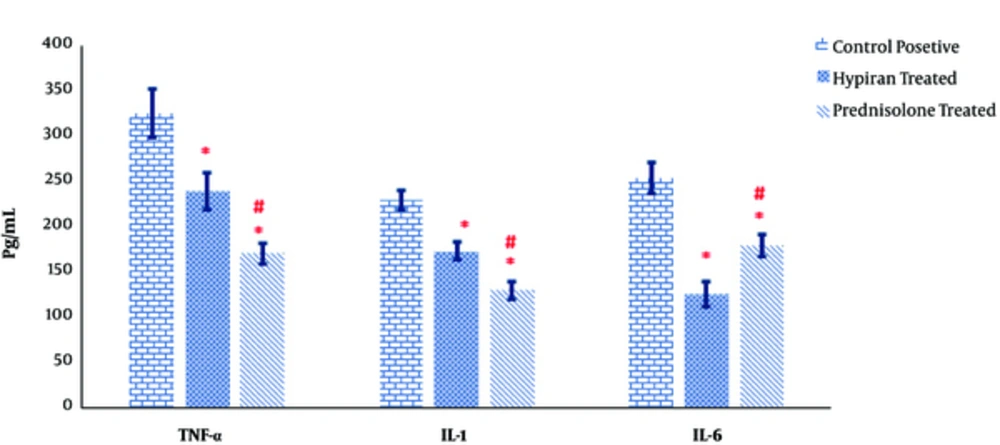
As shown in Figure 5, both medications in rats with colitis resulted in a significant reduction in production of pathogenic inflammatory cytokines TNF-α, IL-1β and IL-6 in the colonic homogenate of colitis rats compared to positive control rats. Albeit, the levels of TNF-α and IL-1β were down-regulated in the guts of Prednisolone treated rats more than Hypiran groups. Nevertheless, Hypiran decreased the levels of IL-6 more profoundly than Prednisolone (Figure 5).
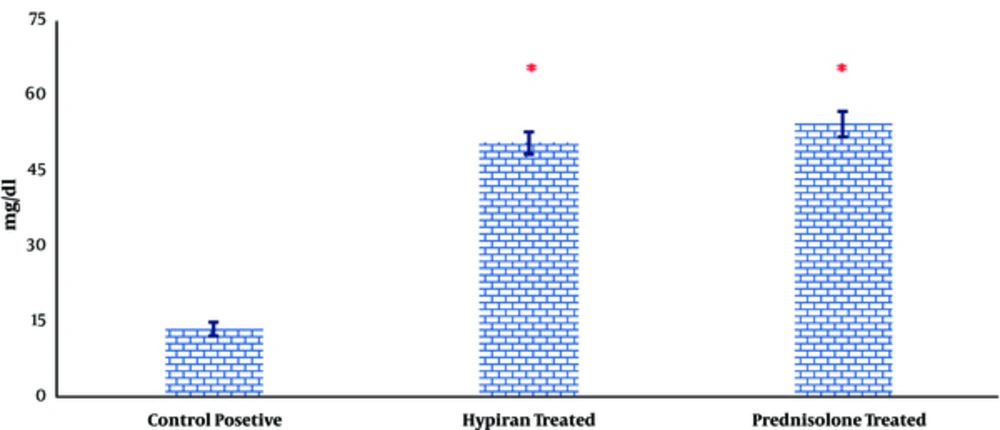
Attained data also showed that both therapies could equally increase the level of total protein in the colonic homogenate compared to colitis rat without any medication (Figure 6).
4. Discussion
Luminal instillation of acetic acid can produce diffuse inflammation that eventually results in erosions and ulcerations of the rat colon. The inflammation can be extended into the lamina propria submucosa, or external muscle layers [30]. After the initial epithelial damage by acetic acid, mucosal and submucosal followed by activation of arachidonic acid pathways and leukocyte migration especially neutrophils [31]. The propagation of the primary inflammation has a noxious effect on the healing process because the increasing swelling and inflammation can promote secondary damage to the already-injured epithelium. The anti-inflammatory drugs like 5-aminosalicylic acid and glucocorticoids used in order to treat inflammation of ulcerative colitis [32, 33]. However, their adverse reactions and side effects and high relapse rate limited their use for alleviation of ulcerative colitis during prolonged treatment [32, 33]. Nowadays, medicinal plants with anti-inflammatory properties are desirable to substitute chemical anti-inflammatory therapeutics [34, 35].
Interestingly, the immunomodulatory and anti- inflammatory properties of H. perforatum were previously documented [10]. Cyclooxygenase (COX) 1 and 2 are two isoenzymes that participate in the initiation of inflammation, therefore, inhibition of these enzymes can regress the symptoms of inflammation [36]. It has been shown that hyperforin, a major component of the H. perforatum can inhibit COX-1 around 3-18 times similar to aspirin [37]. On the other hand, it has been documented that the COX-2 activity and production of inflammatory mediators by lipopolysaccharide-activated macrophage can be decreased by an ethanolic extract of H. perforatum or 4 compounds of this extract (pseudohypericin, amentoflavone, quercetin, and chlorogenic acid) [25]. It is proposed that the decrease in the activation of suppressor of cytokine signaling 3 protein (SOCS3) may partially participate in the reduction of COX-2 activity [38]. In this regard, obtained data in the present survey indicate that Hypiran, as much as Prednisolone, could regress the inflammation and mitigate clinical score induced after intra-luminal instillation of acetic acid in the colons of the Wistar rats. It is important to note that Prednisolone is a potent immunosuppressive drug with a lot of side effects [33]. Conversely, Hypiran is a safe product with immunomodulatory benefits [10].
The production of inflammatory cytokines such as TNF-α, IL-1β and IL-6 can promote and propagate the inflammatory reaction and injurious manner [39]. The attained data showed that the levels of TNF-α and IL-1β were down-regulated in the gut homogenate of Prednisolone treated rats more than Hypiran groups. Nevertheless, Hypiran decreased the levels of IL-6 more profoundly than Prednisolone. Interestingly, it is clear that patients with depression disorders show increased levels of pro-inflammatory cytokines like IL-6 and H. perforatum extract may have beneficial effects in the treatment of depression via decreasing the levels of IL-6 and altering the neuro-mediators [10, 40].
After initiation of inflammation, the emigrant cells like neutrophils can produce a lot of reactive oxygen and nitrogen metabolites [31]. Myeloperoxidase, a peroxidase enzyme, is most abundantly found in neutrophil granulocytes and produces hypohalous acids during the neutrophil’s respiratory burst and reactive oxygen production [25]. The excessive uncontrolled or inappropriate production of radical species may participate in the potentiation and prolongation of inflammatory injuries [41]. The pervious evidences demonstrated that H. perforatum extract could decrease the expression of inducible nitric-oxide synthase [38, 42] and inhibit the LPS-induced production of nitric oxide) in activated macrophages [38]. Also, it has been shown that the treatment of mice by hydro-alcoholic extract of H. perforatum could reduce the potential of reactive oxygen metabolite production by inflammatory cells [10]. The results of this study showed that both therapies with Hypiran and Prednisolone can suppress the levels of oxygen and nitrogen reactive substances. However, the Hypiran has more profound effects on the reduction of nitric oxide compared to Prednisolone, whereas Prednisolone has more impact on the reduction of reactive oxygen metabolites than Hypiran.
Due to participation of free radicals in pathogenesis of ulcerative colitis, eliminating these causes inflammatory triggers may be a useful strategy. In this regard, a previous study showed that vitamin E, as an antioxidant, had therapeutic effects to control the ulcerative colitis [30]. Interestingly, it is evident that ethanolic extract of H. perforatum contains many antioxidant phenolic compounds such as hypericin, hyperforin and their derivatives, rutin, hyperoside, quercetin, chlorogenic acid, flavanols and flavones [43]. The level of the lipid peroxidation in tissue homogenate can be detected by estimation of malondialdehyde level in the tissue samples [27]. Our results showed that the level of MDA was decreased in Hypiran treated group more than Prednisolone treated group. Therefore, some of the beneficial effects reported in the current study may be due to the potent anti-oxidant properties of Hypiran. Of note, unlike Hypiran, Prednisolone doesn’t have any direct antioxidant effects. Therefore, the reduction of MDA in Prednisone treated rats was indirectly occurred by suppuration of reactive oxygen and nitrogen metabolites.
It is reported that total protein levels in colonic tissues were decreased due to intraluminal instillation of acetic acid, which eventuated to the erosions, followed by an inflammation which destroy the cellular macromolecules in colon and disrupt the cell integrity and mucosal recovery in the colonic tissues [24]. Attained results in this study suggest that concurrent with reduction of disease activity index and inflammatory mediators, both medications can reverse the rate of reduction in total protein levels in colonic tissues and therefore promote tissue healing.
After luminal instillation of acetic acid and mucosal damage formation, bacterial microflora can participate in the propagation of inflammation and mucosal damage in the rat colon [44]. Bacterial microflora can also participate in the pathogenesis of ulcerative colitis in humans [45-47]. Interestingly, a large number of previous studies indicated that H. perforatum extracts, especially alcoholic ones had remarkable anti-bacterial properties [48]. Therefore, in addition to the direct anti-inflammatory effects, some beneficial effects of Hypiran in the current study may be the result of direct anti-microbial effects of this extract.
As mentioned above, the intrarectal administration of acetic acid mimics the ulcerative colitis form of IBD. TNBS-induced colitis is another animal form of IBD that induces a chronic phenotype mimicking Crohn’s disease [18]. Similar to our results, an interesting previous study reported that daily intra-peritoneal administration of extract of H. perforatum had a protective beneficiary on TNBS-induced colitis, due to an anti-inflammatory and antioxidant mechanism [49]. A former study reported that colitis rats orally received 300 and 600 mg/kg H. perforatum extract and/or intrarectally treated with 10 and 20% gel form of H. perforatum extract for 7 days, showed significant histopathological healing concurrent with a significant reduction in tissue MDA levels [50]. Similarly, current results showed that H. perforatum even at a lower dose (150 mg/kg) has a significant healing benefit in colitis rats.
As a conclusion, this data suggests that the Hypiran as a hydro-alcoholic extract of H. perforatum may be used as a natural source to alleviate the signs of ulcerative colitis in a rat model. Moreover, the beneficial effects of Hypiran may be partly due to inhibition of inflammatory cytokines (TNF-α, IL-1β and IL-6) and the levels of malondialdehyde, nitric oxide and MPO-activity in the inflamed gut. Nevertheless, other mechanisms may also be involved and these remain to be clarified.