1. Introduction
The emergence of nano technology has provided an extensive research in recent years by intersecting with various other branches of science and forming impact on all forms of life [1]. Nanoparticles have expressed significant advances owing to wide range of applications in the field of bio- medical, sensors, antimicrobial, catalysts, and electronics, optical fibers, agricultural, bio-labeling and in the other areas. The synthesis of metal nanoparticles is a more important research branch in nano technology. Chemical procedures are the most widely used methods for synthesis of metallic nanoparticles [2]. With the development of new chemical or physical methods, the concern for environmental contaminations are also heightened as the chemical procedures involved in the synthesis of nano materials generate a large amount of hazardous byproducts. Thus, there is a need for ‘green chemistry’ that includes a clean, nontoxic and environment-friendly method of nanoparticles synthesis [3].
In the few past years, the potential of various plants for the synthesis of silver nanoparticles (SNPs) was explored. Several plants like rose, banana, hibiscus, geranium leaves, Cinnamomum, Aloe Vera, basil tansy fruits, and sweet pepper were used to synthesize nanoparticles [3-11]. The biological approaches to synthesis of nanoparticles are better than chemical and physical procedures because of low energy and time expenditure. This method requires no toxic solvents and no dangerous material for environment. Green synthesis of nanoparticles is an eco-friendly method and uses natural solvent [12]. Nanoparticles produced by plants are more stable, and the rate of synthesis is faster than that in the case of other organisms. Moreover, the nanoparticles are more various in shape and size in comparison with those produced by other organisms [13].
The aim of this study was to investigate antibacterial activity of silver nanoparticles synthesized by using extracts of Hedera helix against Bacillus subtilis and Klebsiella pneumoniae.
2. Methods
2.1. Reagent and Materials
All materials used in this experimental study including Nutrient broth media, Meuller- Hinton agar media,and silver nitrate for the synthesis of nanoparticles were purchased from Merck company in Germany. Standard strain of the Bacillus subtilis (ATCC 1715) and Klebsiella pneumoniae (ATCC 1290) were purchased from the Center of Scientific and Industrial Research of Iran.
2.2. Preparation of Hedera helix Extract
Hedera helix leaves were collected from areas that had grown wild. The leaves were washed several times with distilled water to remove the dust particles. The leaves were cut into small pieces and 10 g were boiled in a 500-mL glass beaker along with 50 mL of sterile distilled water for 20 minutes. The extracted was concentrated by evaporation. After boiling, the colour of the aqueous solution changed from watery to yellow colour. The aqueous extract was separated by filtration with Whatman No. 40 filter paper. The leaf extract was stored at room temperature to be used for biosynthesis of silver nanoparticles from silver nitrate.
2.3. Synthesis of Silver Nanoparticles
The source of silver was silver nitrate (1 mM) in distilled water. Silver nitrate solution was prepared and was reduced using Hedera helix extract at room temperature.
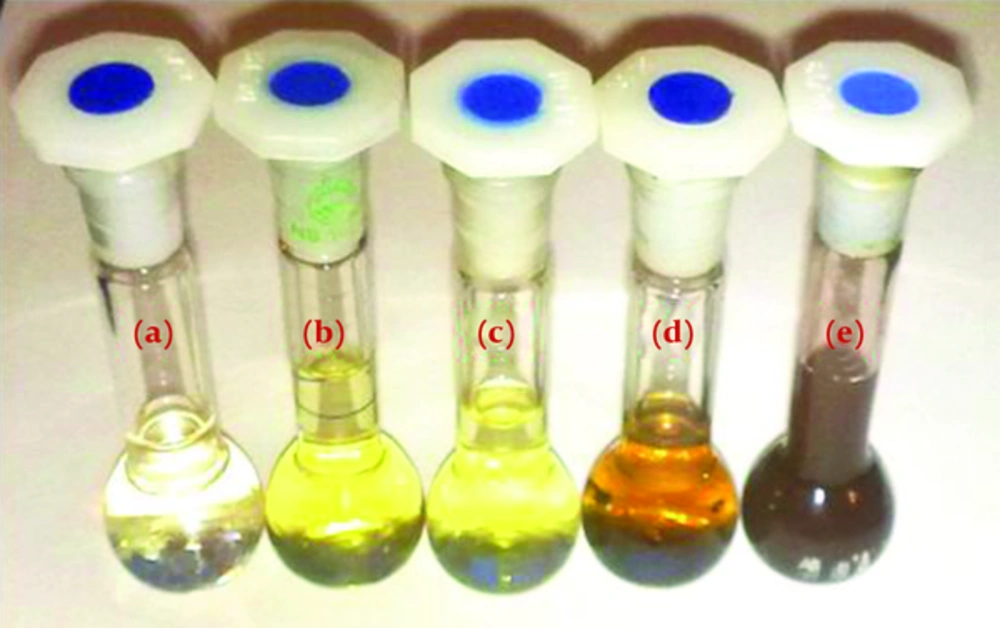
The system was stirred and reduction took place rapidly at room temperature under sun light and completed in 15 minutes. During the bio reduction, the chemical reaction takes place resulting in color change in the reactants from pale yellow into dark brown. The appearance of brown color indicates formation of silver nanoparticles. A picture from reaction mixtures and color changing is shown in Figure 1. The formation of AgNPs was furthermore confirmed by spectroscopic analysis, TEM and DLS techniques.
2.4. Characterization of Silver Nanoparticles
2.4.1. UV-Visible Spectroscopy
The formation and completion of silver nanoparticles was characterized by UV-visible spectroscopy using a Double beam spectrophotometer (Perkin Elmer lambda 15). The bio reduction of silver ions in aqueous solution was monitored by UV-VIS spectra of the solution between 360 nm to 600 nm.
2.4.2. TEM Analysis
Transmission electron microscopy (TEM) technique was used to visualize the morphology of the Ag NPs. The 80 kV ultra-high-resolution transmission electron microscope (Zeiss- EM10C). Prior to being loaded into the TEM, the sample was made by grinding using mortar and dropped into a wholly carbon-coated copper grid.
2.4.3. DLS Analysis
To confirm the size distribution of the silver nanoparticles (DLS) (Malvern Instruments, MAL1001767) technique was applied
2.5. Anti-Bacterial Assay of Silver Nanoparticles
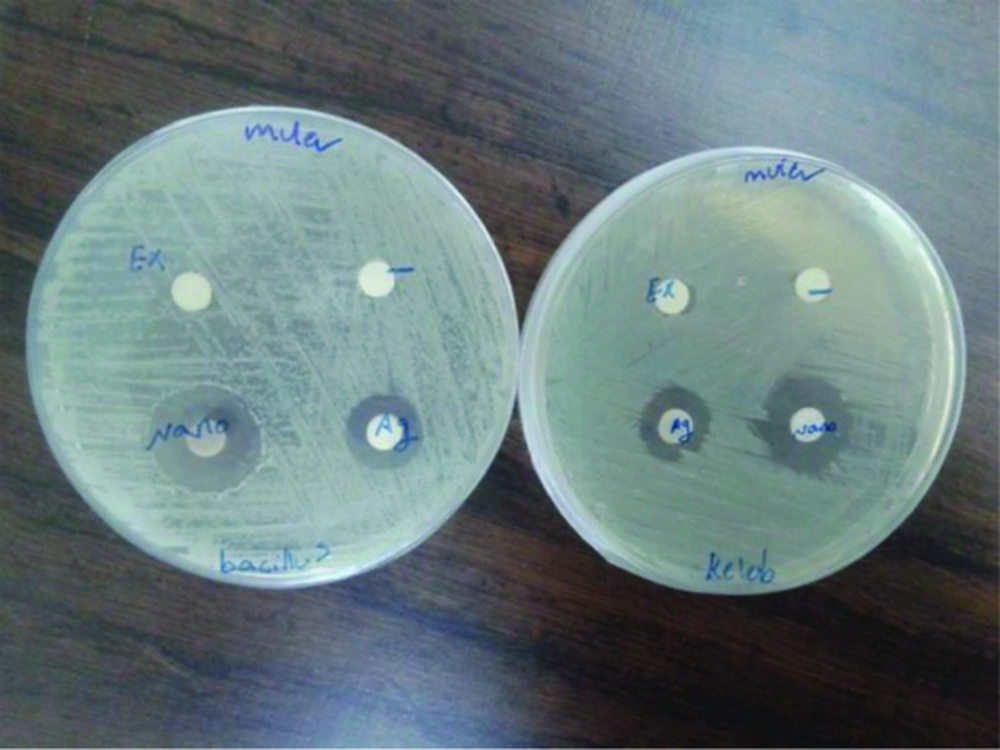
The produced colloid of silver nanoparticles was evaluated for antibacterial activity. Disc diffusion method [13] was used. The discs were soaked with double distilled water, Hedera helix extract, silver nitrate solution and solution containing silver nanoparticles. Then the discs were air dried in sterile condition. Standard strain of the Bacillus subtilis (ATCC 1715) and Klebsiella pneumoniae (ATCC 1290) were selected for the investigating. Pure cultures of bacteria as 0.5 macfarland (108 Cfu/mL) was swabbed on the Muller-Hinton agar plates.
Plates containing media as well as culture were divided in to four equal parts and previously prepareddiscs were placed on each part of the plate. The discs were placed in the following order: disc soaked with double distilled water as negative control, disc soaked with Hedera helix extract, disc soaked with 1 mM silver nitrate solution and disc soaked with solution containing Hedera helix extract mediated synthesized silver nanoparticles. The plates were incubated at 37°C for 24 hours. Then, the maximum zone of inhibition were observed and measured for analysis against each type of test microorganism.
3. Results
3.1. UV Visible Spectroscopy
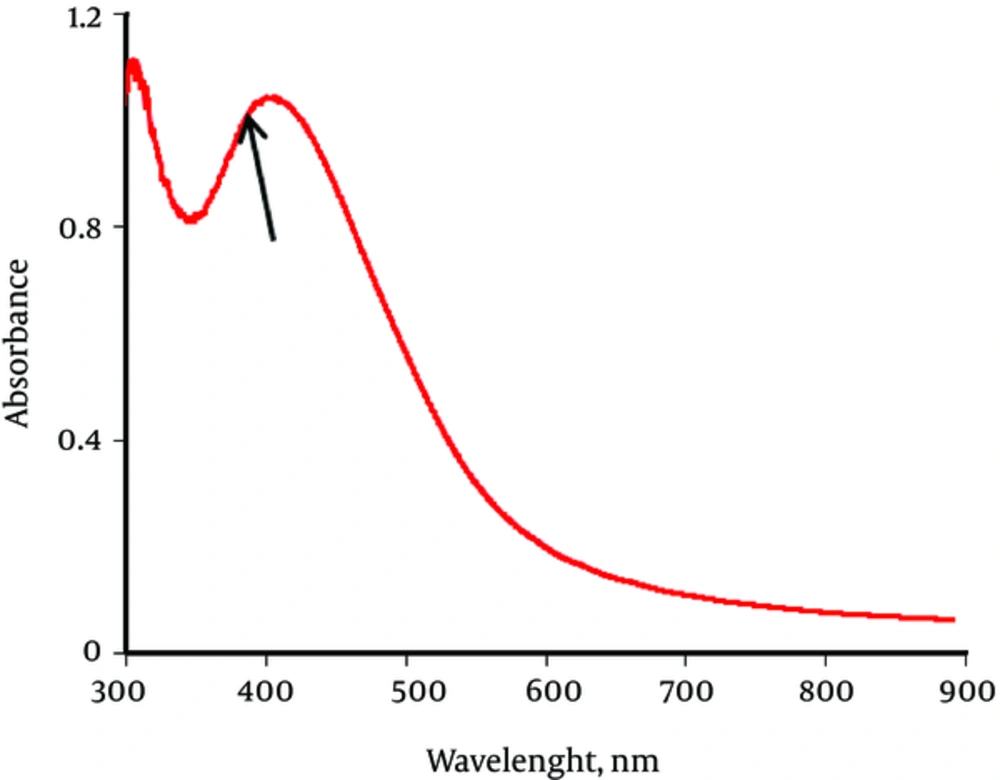
In order to confirm the formation of silver nanoparticles, UV Visible Spectroscopy was employed. The UV Visible pattern in Figure 2, shows the characteristic Surface plasma resonance (SPR) of colloidal silver nanoparticles ranges between 300 nm to around 600 nm and exact peak is at 431 nm.
3.2. TEM Analysis
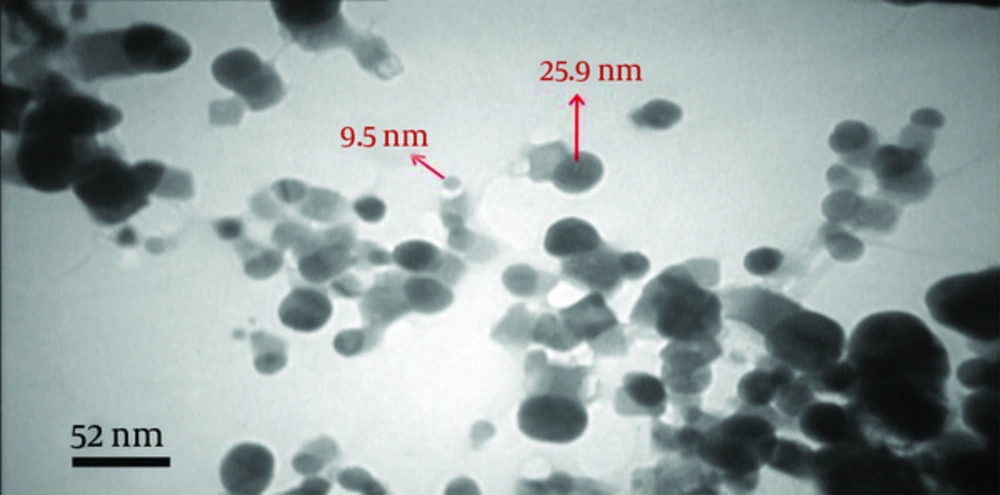
Figure 3, Shows the transmission electron microscopic (TEM) image of colloidal silver nanoparticles. The TEM image indicated the clustered nature of the particles and the surfaces of the aggregates are rough. The particles are more or less spherical in shape-sizes and range from 10 - 30 ± 2 nm in diameter.
3.3. DLS Analysis
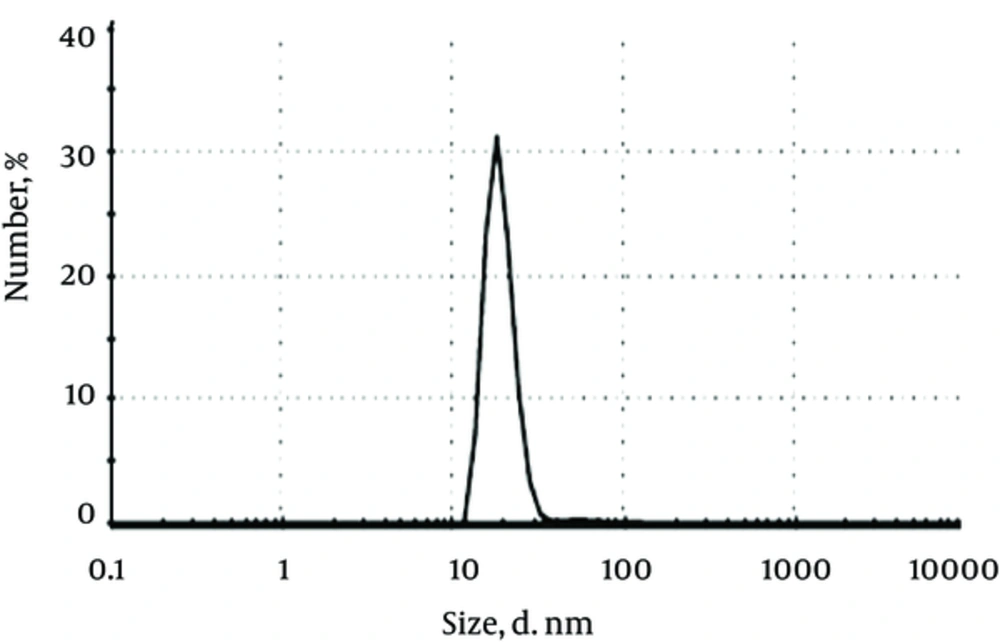
Figure 4, presents the histogram of particles size distribution obtained by the DLS analysis for colloidal silver nanoparticles. The particle sizes show a narrow range of distribution and proved an average diameter of 20 - 25 ± 1 nm for silver nanoparticles.
3.4. Antibacterial Activity
Antibacterial property analysis In this study, the antimicrobial property of AgNPs were investigated by growing B. subtilis and K. pneumoniae colonies on Muller-Hinton agar plates supplemented with AgNPs. Results obtained in previous studies also support the antibacterial potential of AgNPs. In the Muller-Hinton agar plate the zone of inhibition was observed around both the disc (AgNO3 and AgNPs) as shown in Figure 5.
In both cases (AgNO3 and AgNPs) inhibition zone for gm+ veB. subtilis and gm - K. pneumonia were equal. But, in comparison to AgNO3 and AgNPs, The zone of bacterial inhibition by AgNPs was more than AgNO3. No zone of inhibition was obtained in case of control and Hedera helix extract.
4. Discussion
The bio reduction approach toward the synthesis of silver nanoparticles is also quite eco-friendly when compared to existing chemical and physical methods. In the present study, it is found that Hedera helix extract can also be a good reducing agent for synthesis of silver nanoparticles and its related kinetics would help to synthesis desired size ranges of nanoparticels to suite a variety of applications. The colloidal silver nanoparticles were studied using different characterization techniques such as Uv-vis spectroscopy, TEM and DLS. The resulted of colloidal silver nanoparticles were evaluated for antibacterial activity. The colloid of silver nanoparticles has got enhanced antibacterial activity against Gram-positive (B. subtilis) and Gram-negative (K. pneumonia), bacteria. In 2010 Bylka et al. introduce Hedera helix as medicinal plant and Ivy leave extracts exhibit spasmolytic/antispasmodic, anti-inflammatory, antimicrobial, analgesic, anthelmintic, antitrypanosomial, antileishmanial, antitumor, antimutagenic, moluscocidal, antioxidant and antithrombin activities [14]. In 2001 Hussain et al. used cytotoxic potential of Hedera helix leaves [15]. Due to the development of antibiotic resistance and the outbreak of infectious diseases caused by resistant pathogenic bacteria, the researchers are now searching for new unconventional antibacterial agents. Ansari et al. in 2011 Examined antibacterial effect of silver nanoparticles (synthesis by chemical methods) against Staphylococcus aureus and results showed that AgNPs exhibit bacteriostatic and bactericidal effect towards all clinical isolates [16]. Also, Sadeghi et al. 2011 investigated antibacterial activity of silver nanoparticles (synthesis by chemical methods) against S aureus and E coli and results showed that the antibacterial activity against E coli is lower than that against S aureus, probably because of the difference in cell walls between gram positive and gram negative bacteria [17]. The results of this study are in agreement with our studies.
In 2014 Zarei et al. tested antibacterial effect of silver nanoparticles (synthesis by chemical methods) against four foodborne pathogens (Listeria monocytogenes, Escherichia coli, Salmonella typhimurium and Vibrio parahaemolyticus) that silver nanoparticles showed great antibacterial effectiveness on four important foodborne pathogens [18].
But in this research silver nanoparticles synthesis by chemical methods that is harmful to the environment. Recently green methods are used for synthesis of Nanoparticles. Mallikarjuna et al. in 2010 synthesis of silver nanoparticles using Ocimum leaf [19]. Mohaseli et al. (2015) synthesis of silver nanoparticles with green method by using Sesame seeds extract [20].
Also the results of search by Xia in 2010 indicated that the ivy nanoparticles were more efficient in blocking UV light, less toxic to mammalian cells, easily biodegradable, and had a limited potential to penetrate through human skin [21]. Parvu et al. (2015) searched on phenolic compounds and antifungal activity of Hedera helix (Ivy) flowers and fruits. The results showed the antifungal activity of the fresh flower extract was stronger than that of the fresh fruit extract and was compared to that of an antimycotic drug [22].
Research on antibacteriali effect Hedera helix extract in Iran performed by Hooshyar et al. in 2014 worked on therapeutic effect of Hedera helix alcoholic extract against cutaneous leishmaniasis caused by leishmania major in Balb/c Mice [23]. But, in this study antibacterial activity of silver nanoparticles synthesized by using extracts of Hedera helix searched. This is an interesting report on the antibacterial effect of colloidal silver nanoparticles that was prepared with green synthesis method. It may be investigated as potential candidate for request in futuristic biomedical fields. However our findings could be targeted for the promising potential applications including biosensing devices, and nanoelectronic because of its pollution free and eco –friendly approach. This green synthesis approach shows that the environmentally bengin and renewable leaf extract of Hedera helix can be used as an effective stabilizing as well as reducing agent for the synthesis of silver nanoparticles. Therefore, AgNPs could be a good alternative for cleaning and disinfection of equipment and surfaces in food-related environments.