1. Background
The rhizome of Zingiber officinale Rosecoe (ginger) which known as a food spice was a herbal medicine used around the world for centuries. Gingerols (i.e. 6-gingerol, 8-gingerol and zingerone) have been recognized as the main active ingredients of ginger, which is accountable for its pungent taste. Other constituents include volatile oil, aryl alkanes, shogaols, diarylheptanoids and starch [1]. Different therapeutic effects of this plant reported previously. Several studies showed various pharmacological effects of this compound including anticancer [2], anxiolytic [3], anti-inflammatory [4], antithrombotic [5], hypoglycemic and hypolipidemic [6] effects. Ginger also plays a major role in gastrointestinal tract such as cholagogic effect [7], anti-inflammatory [8], antineoplastic [9, 10] anti-helicobacter pillory, anti-emetic and prokinetic [11-15] effects. In digestive tract, it has been demonstrated that ginger extract abolished the chemotherapy induced delay in gastric emptying in rats [16] and also it reduced nausea associated with motion sickness [17]. Moreover in rat colon it has been shown that ginger extract decrease motility [18]. While in the rat ileum it has spasmolytic effect [19], in guinea pig ileum ginger improves motility [20]. Isolated gingerols and shogaols have been shown to modify gastric motility in animal experiments [21]. Although it was demonstrated that ginger accelerated gastric motility [12], about lower gastrointestinal region there are paradoxical information [18-20]. It seems that the effect of ginger in gastrointestinal tract motility, depends to organ and spices and different in upper and lower part of gastrointestinal tract. On the other hands, changes induced in small intestine motility, can affect on this function. Despite of different reports about the pharmacological effects of ginger in human and animal studies, there are few reports of ginger effect on ileum motility and underlying mechanism in literature.
2. Objectives
Therefore, given the widespread use of ginger for the treatment of gastrointestinal diseases, in the present study we have investigated the effect of this herbal medicine and underlying mechanism on pre-contracted rat ileum segments.
3. Materials and Methods
3.1. Preparation of Extract
In this experimental study, the rhizome of ginger, for the proposed work was obtained from local herbal stores in Zahedan and identified with herbarium of Sistan and Baluchistan university. The whole plant material was dried under shade and mechanically reduced to powder and stored in air tight containers for further use in extraction process. The ginger powder was successively extracted using soxhlet apparatus with methanol 70% (1 to 4 portions) as solvent. The extracts were dried in incubator at 37ºC. The dried methanolic extract was dissolved in saline solution for further experiments in 100 or 200 µg/mL of organ bath.
3.2. Animals
Forty male Wistar albino rats (Zahedan university strain) (220 - 250 g) were maintained under controlled conditions of temperature (22 - 24ºC) and 12 hour of dark and light cycle until used and divided in five groups randomly (n = 8 in each group). Groups were consisting: ginger alone (100 and 200 µg/mL), KCl + ginger (100 and 200 µg/mL), carbachol (100 μmol/mL) + ginger (200 µg/mL), pretreated with L-NAME (300 μmol/mL) and KCl + ginger (200 µg/mL), pretreated with verapamil (100 μmol/mL) and KCl + ginger (200 µg/mL). The rats had free access to water and food. All procedures were carried out in accordance with Zahedan University of Medical Sciences guidelines for animal care. Animals were killed by decapitation and the whole intestine was removed and immediately placed in freshly prepared Tyrode’s solution (composition mM: NaCl = 136.9, KCl = 2.68, MgCl2 = 1.05, CaCl2 = 1.8, NaHCO3 =11.9 and glucose = 5.55) at room temperature. The mesentery and fatty tissue were removed and the intestine was emptied of its contents by flushing Tyrode’s solution. Two centimeter long segments taken from the ileum was connected vertically to a tissue holder by cotton threads and to isometric transducer ML T0 50/D. The tissues were bathed in a 25 mL water-jacketed organ bath containing 20 mL Tyrode’s solution and placed under 0.5 g tension. The Tyrode’s solution was maintained at a temperature of 37 ± 0.5ºC. Each tissue was left to equilibrate for 1 hour and washed every 15 minutes. Longitudinally-mediated responses were recorded using isometric transducer ML T0 50/D and displayed, stored and analyzed in a PC Pentium computer using Power Lab Chart V4.0.4 software. Each experiment was repeated for 8 times, using fresh tissues. Therefore, for each part of experiment 8 animals have been used. The final concentrations of ginger extract were 100 and 200 μg/mL in organ bath. After a minimal 60 minutes equilibration period the tissues were subjected to KCl 120 μmol/mL. After stable control contractions by KCl had been recorded, the responses were observed in the presence of ginger (100 and 200 μg/mL).
The contact time for each concentration of ginger was 10 minutes. Preliminary experiments showed that ginger reached its maximal inhibitory effect within this time period. In some experiments, the effect of verapamil (100 μmol/mL), a calcium channel blocker, on KCl induced contractions was evaluated. The effect of ginger was also evaluated after the administration in the bath (contact time 30 minutes) of verapamil (100 μmol/mL) and NG-nitro-L-arginine methyl ester (L-NAME 300 μmol/mL) (to block NO synthesis). In preliminary experiments the effect of atropine (1 μmol/mL) on KCl induced contractions was evaluated after a contact time of 30 minutes. The effect of ginger was also evaluated on the contractions produced by carbachol (100 μmol/mL). This concentration of carbachol gave a contractile response which was similar in amplitude to that obtained by KCl.
3.3. Drugs
Atropine, carbachol, L-NAME and verapamil were purchased from Sigma (UK), KCl and other reagents were obtained from Merck company (Germany). Ginger methanolic extract was dissolved in saline solution. The other drugs were dissolved in distilled water.
3.4. Analysis of Results
Current investigation was an experimental study, based on changes in g tension of isolated tissues to KCl before and after different treatments. The tension was expressed as the percentage of the maximal response to KCl ± standard error of means (SEM). The significance of differences was determined by ANOVA statistical test, using SPSS-17 software. Values less than 0.05 were considered statistically significant.
4. Results
4.1. The Effect of Ginger Extract on Contractile Response Induced by KCl and Carbachol
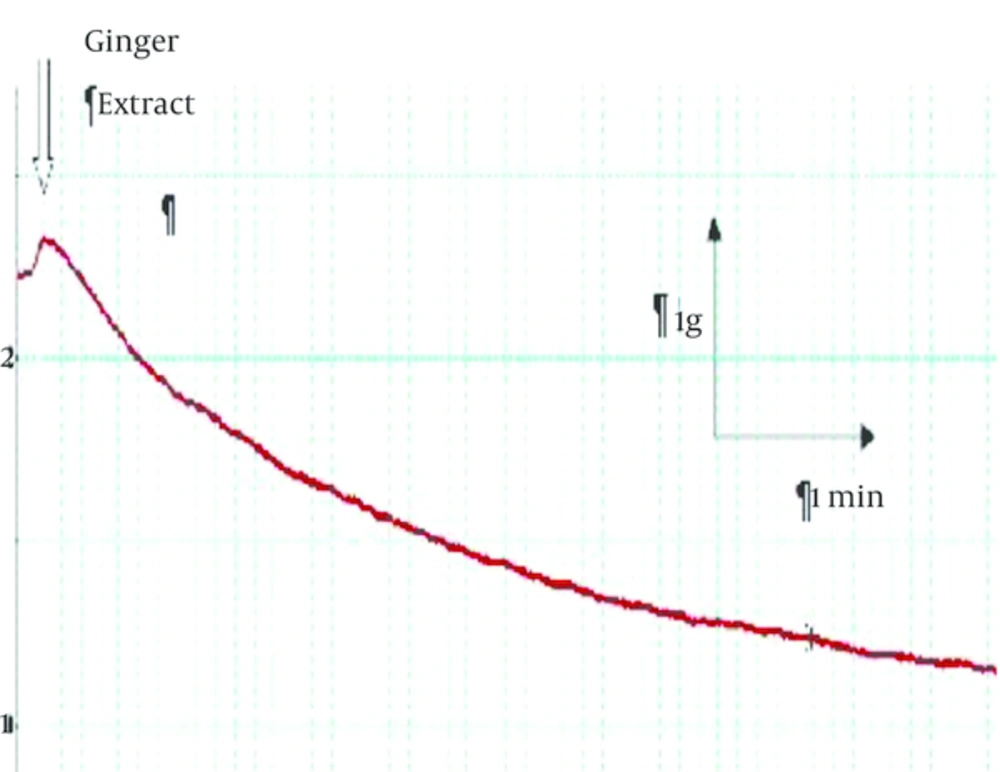
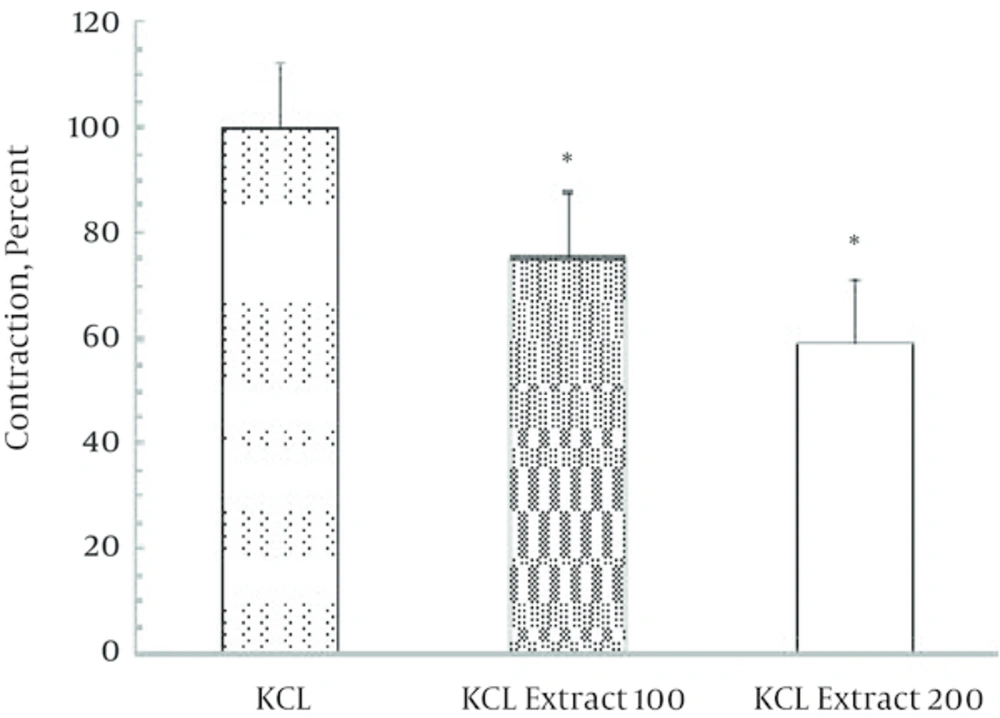
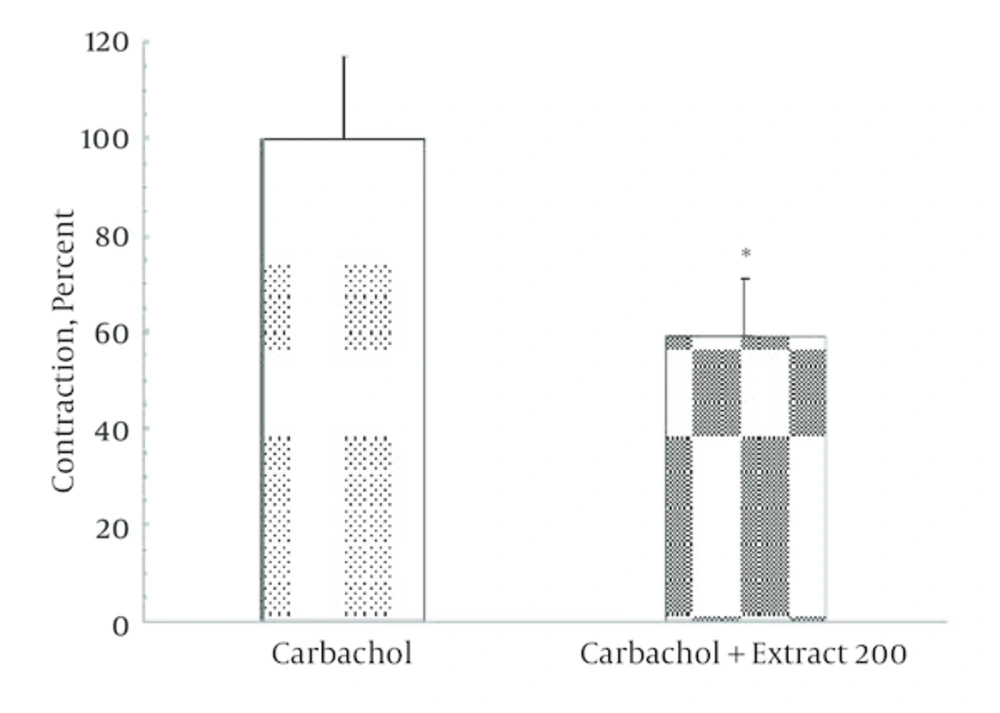
Non-cumulative concentrations of ginger extract (100 and 200 μg/mL) significantly decreased (P = 0.027) the contractile response induced by KCl (120 μmol) in a dose dependent manner (Figures 1 and 2). In Figure 1 representative trace showing that injection of 200 µg/mL ginger extract induced a small contraction (0.1 g) followed by significant relaxation (2 g). According to Figure 2, this reduction in contractile response has been significantly increased by elevating of ginger concentration. Moreover, contractile response induced by carbachol (a cholinergic agonist) 100 μmol significantly reduced to 60% in the presence of ginger extract (Figure 3).
4.2. The Effect of L-NAME on Relaxation Induced by Ginger Extract on Pre-Contracted Tissues
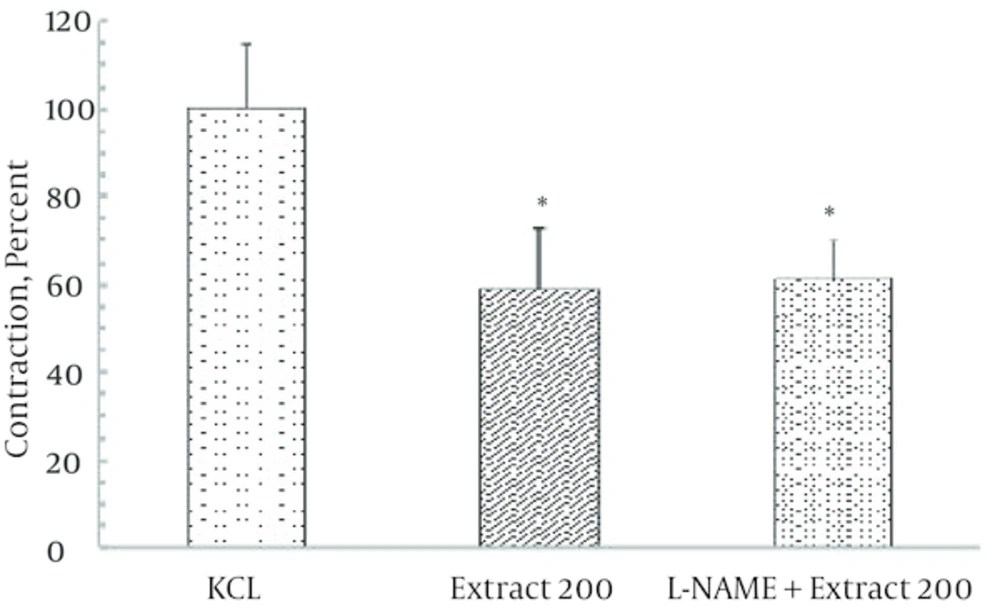
Incubation of ileal tissues by L-NAME 300 μM, a nitric oxide synthase inhibitor failed to modify the contractile response induced by KCl alone and in the presence of ginger extract (200 µg/mL) (Figure 4).
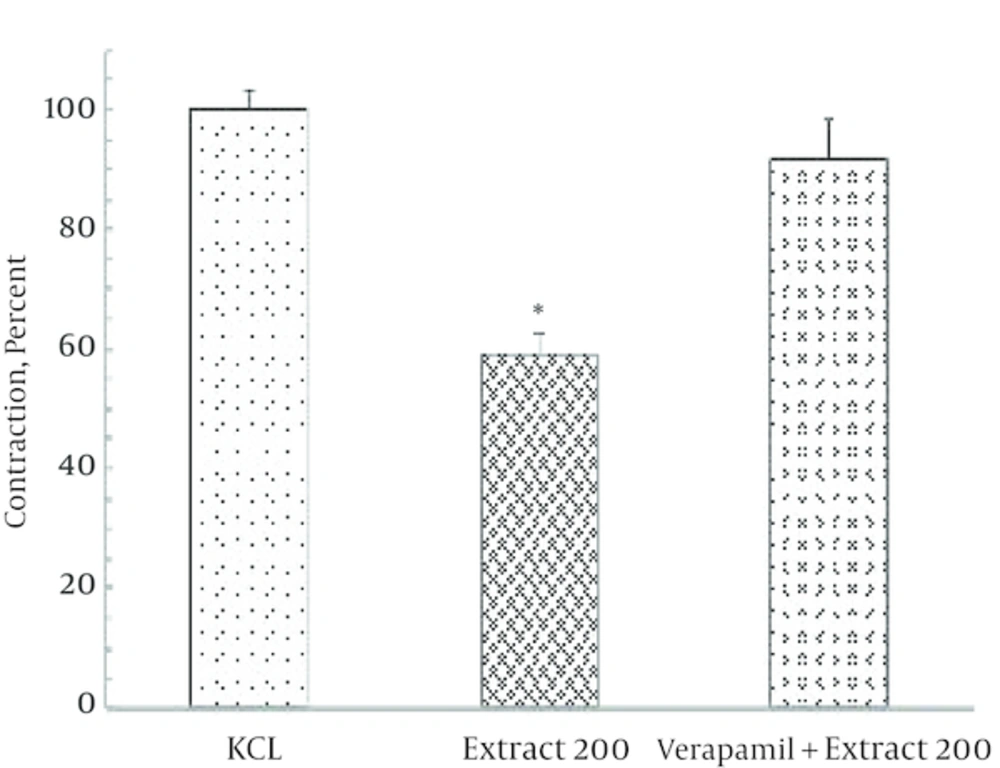
4.3. The Effect of Verapamil on Contractile Response to KCl in the Presence of Ginger Extract
In the presence of verapamil the KCl contractile response decreased by 50% but not abolished. Moreover in the presence of verapamil, ginger extract failed to modify the contractile response to KCl (Figure 5).
5. Discussion
In present study, it was shown that hyrdoalcholic extract of ginger is capable to reduce the contractile response to KCl and carbachol which indicates a relaxation response. This relaxation response do not inhibit with L-NAME. Thus this relaxatory effect of ginger not mediated with nitric oxide. Also, these results shown that ginger extract can to reduce the contraction induced with carbachol in rat ileal smooth muscle.
Effects of ginger on the gastrointestinal motility were widely reported [15, 16, 20]. It was demonstrated that ginger accelerated gastric motility such as emptying and antral contractions in healthy volunteers and in patients with functional dyspepsia [12]. However about lower gastrointestinal region there are controversial reports [18-20]. In line with present study, Borrelli et al. demonstrated that ginger could inhibit both electrical field stimulation (EFS) and acetylcholine-evoked contractions [22]. However, another study presented that even single dose of ginger dried root was able to increase carbachol-induced small intestinal transit [20]. Borrelli et al. concluded that ginger possesses both prejunctional and postjunctional inhibitory effects on rat ileal contractility; the prejunctional inhibitory effect of ginger on enteric excitatory transmission could involve a capsazepine-sensible site which could be vanilloid receptors [22]. However they pre-contracted rat ileum by electrical field stimulation which makes different experimental condition. Furthermore in current study relaxation response induced by ginger extract was insensitive to L-NAME, NO synthase inhibitor. This indicates that relaxation response was not mediated by nitric oxide pathway which is the most important inhibitory neurotransmission in gastrointestinal tract [23]. To find out mechanism underlying relaxation response, the mode of relaxation of verapamil, an L-type calcium channel blocker was compared with ginger extract which showed similar trend and also in the presence of verapamil, ginger extract failed to attenuate the contractile response to KCl. It was already shown that the predominant source of Ca2+ for the contractile response is extracellular Ca2+ and intracellular Ca2+ has little role to play in mediating excitation-contraction coupling in rat distal colon smooth muscle in vitro [24].
In 2006, Kong et al. demonstrated that verapamil, significantly attenuated, but did not completely eliminate the high K+- and ACh-induced contraction, indicating that additional channels might be involved in the contractile mechanism. They concluded that contraction of smooth muscle in the distal colon is regulated by multiple Ca2+ channels. In addition to voltage-operated Ca2+ channel -mediated Ca2+ influx, store-operated Ca2+ channels participates in contractile response of distal colon smooth muscle in rats [25]. In conclusion, present study showed that ginger hyrdoalcholic extract have inhibitory effect on contractile response induced by either carbachol or KCl. However this relaxation response could not be mediated by nitric oxide. Furthermore, disappearing of relaxation response in presence of verapamil indicates that voltage gated L-type calcium channel might mediate the ginger relaxation response on pre-contracted ileal tissues.
Results of this study shown that hydroalcholic extract of ginger can decrease contraction induced with KCl and carbachol in rat ileal muscle and nitric oxide has no any involvement in this effect. Further experiment using electrophysiological study is necessary to investigate the exact role played by Ca2+ channels in rat ileum or other mechanism involved in relaxation response induced by ginger.