1. Background
Many efforts have been done to discover new antimicrobial compounds from various kinds of sources such as soil, microorganisms, animals and plants. One of such resources is folk medicine and systematic screening of them may result in the discovery of novel effective compounds. Further, scientific investigation and information of the therapeutic potential of the plant material is limited. Use of medicinal plants by man has been known for centuries and therapeutic efficacy of several herbal species has been widely described [1]. Fungal diseases are often caused by fungi that are common in the environment. Fungi live outdoors in soil and on plants and trees as well as on many indoor surfaces and on human skin. Most fungi are not dangerous, but some types can be harmful to health [2]. Microfungi of the genus Alternaria are ubiquitous pathogens and saprophytes. Many species of the genus Alternaria commonly cause spoilage of various food crops in the field or post-harvest decay. Due to their growth even at low temperatures, they are also responsible for spoilage of these commodities during refrigerated transport and storage. Several Alternaria species are known producers of toxic secondary metabolites Alternaria mycotoxins. A. alternata produces a number of mycotoxins, including alternariol, alternariol monomethyl ether, altenuene, altertoxin I, altertoxin II, altertoxin III, tenuazonic acid and other less toxic metabolites [3]. Penicillium produces mycotoxins that when ingested or inhaled in large quantities, can cause considerable harm to humans and other mammals [3]. Avicenniaceae family is a member of true mangrove plants, which has one genus, 11 species and several sub species. Avicennia marina is the most current species among these plants in Iranian mangrove forest. A. marina is a mangrove tree species that is extraordinarily adaptable with a wide latitudinal range closely associated with its flexible growth pattern. A. marina grows mainly in the Indo-Pacific region within a latitudinal range of 30°N to 30°S [4]. But another suitable different environment for marine and terrestrial plants could be used for its growing. In general, mangroves are trees, which grow in saline coastal habitats in the tropics and subtropics [5]. The mangrove habitats get food and wide variety of traditional products and artifacts from mangroves. Mangroves are widely used for herbal medicine, e.g. Avicennia illicifolius is used for skin disorders, boils and wounds [4]. A novel alkaloid, acanthicifolin has been isolated from the plant. Mangrove plants are a rich source of steroids, triterpenes, saponins, flavonoids, alkaloids and tannins. Extracts from different mangrove plants are reported to possess diverse medicinal properties such as antibacterial and antihelminthics [6].
2. Objectives
The aim of this study was to determine antifungal effect of the aqueous and ethanolic extracts of A. marina (different concentrations) on Penicillium digitatum and Alternaria citri in vitro.
3. Materials and Methods
This experimental study was conducted at industrial microbiology laboratory, department of food sciences and technology, Ferdowsi University of Mashhad in 2013.
3.1. Plant Material
The leaves of A. marina were collected from the mangrove forests of Qeshm, Iran, which extends from 26°50’N and 56°0’E. Branches and leaves of the plants were cleaned with tap water, dried for 72 hours in appropriate condition in shadow and then filtered using a 60-mesh sieve.
Extract preparation and determination dry weight of extracts: One hundred grams of powdered leaves of A. marina were putted into a flask with 500 mL of ethanol and aqueous and the mixture was then extracted by agitation for 5 hours at 25°C. Then, a maceration of the extracts was done overnight for 24 hours. After, the ethanolic layer containing the extract was taken. The extraction was repeated on the remaining amount of the precipitate using 150 mL of ethanol and all extracts were filtered by using a 0.45 Millipore filter paper. After that, the two fractions of extracts were mixed together and then concentrated using a rotary evaporator at 40°C under reduced pressure (227 m/bar for ethanol). After that, the extracts were stored at -20°C till their usage in the different tests. The extracts resolved in ethanol and distilled water.
3.2. Inoculum Preparation
Molds A. citri and P. digitatum was maintained on Sabouraud dextrose agar (DEFCO Laboratories, USA), sterile distilled water containing 0.05% Tween 80 was added to the surface growth and spores and hyphae were scraped off with a sterile wire loop. Sterile tubes containing ringer solution and its turbidity, was measured by spectrophotometer at 530 nm wavelength. It was diluted by ringer solution until the solution turbidity equalizes with 0.5 McFarland standard solutions. Suspension should have contains 1.5 × 108 CFU/mL [7, 8].
3.3. Screening for Antimicrobial Activity
The method of Collins [8] was used for antimicrobial activity evaluation of the plant extracts. Extract (0.2 g) was reconstituted in 5 mL sterile distilled water and vortexes for homogeneity. One milliliter of the A. marina extract was added to sterile Petri dishes to make a final concentration of 2,000 μg/mL. The plates were prepared in duplicates and allowed to set at room temperature. A loop-full of each organism was streaked on the solidified medium and incubated for 72 hours at 27°C. Control plates comprising to extract without inoculums.
3.4. Antimicrobial Activity
The antimicrobial assay was performed by methods viz, disk diffusion for solvent extract. The Sabouraud dextrose agar (SDA) was inoculated with 100 µL of the inoculums and poured into the Petri dish. For disc agar diffusion method, the disc (7 mm) on SDA (DEFCO Laboratories, USA) plates were saturated with 100 µL of the test compound allowed to dry and was introduced on the upper layer of the seeded agar plate. The plates were incubated 72 hours at 27°C. Microbial growth was determined by measuring the diameter of inhibition zone. For each fungi strain, controls were maintained where pure solvents were used instead of the extract. The result was obtained by measuring the zone diameter. The experiment was done three times and the mean values are presented [9].
3.5. Determination of Minimum Inhibitory Concentration (MIC)
MIC was determined according to agar dilution method [10]. Various concentrations (2, 4, 8, 16, 32, 64, 128, 256 mg/mL) of extract were prepared in 10 cm experimental tubes containing Sabouraud dextrose broth (SDB). Each tube contains 9 mL of SDA and was sterilized in autoclave. After cooling, 1 mL of each extract concentration were added to each tube, to make the final concentrations of 2, 4, 8, 16, 32, 64, 128, 256 mg/mL. The mixture of SDA and extract was poured into plates aseptically in a laminar flow cabinet. After solidification of the agar medium, 2 μL of adjusted spore suspension were added to each plate by micropipette and incubated at 27°C. The SDA without any herbal extract served as control. The MIC was regarded as the lowest concentration of the extract that did not show any visible growth after 12 days of incubation (compared with control).
3.6. Determination of Minimum Fungicidal Concentration (MFC)
MFC was determined according to agar dilution method [11] with slight modifications. The MFC was determined by incorporating various concentrations of extracts (2 - 256 mg/mL) in SD broth in tubes. One milliliter adjusted spore suspension was added to each tube and incubated at 27°C for 3 days. The SD broth without incorporation of dried herbal extract and 1 mL of adjusted spore suspension served as positive control and SD broth alone served as negative control. The tubes which showed no visible growth after 3 days incubation were subculture on extract free SDA plates and incubated at 27°C for 7 days. The MFC was regarded as the lowest concentration of the extract that prevented the growth of any molds colony on the solid medium.
3.7. Statistical Analysis
All the assays were carried out in triplicates. The experimental results were expressed as mean ± SD. The data were analyzed using one way analysis of variance (ANOVA) using SPSS-17.
4. Results
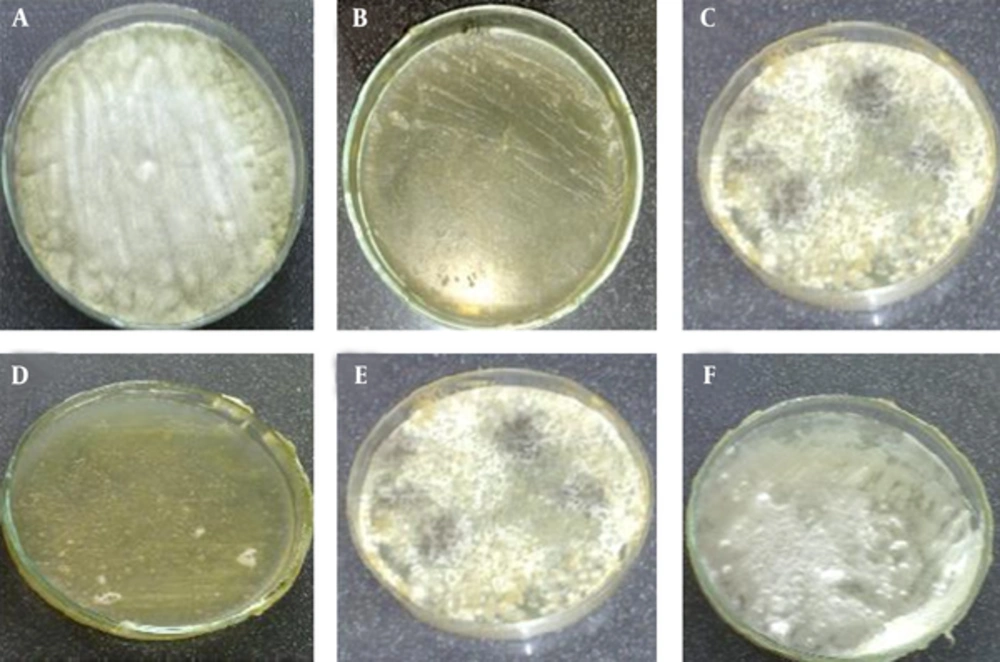
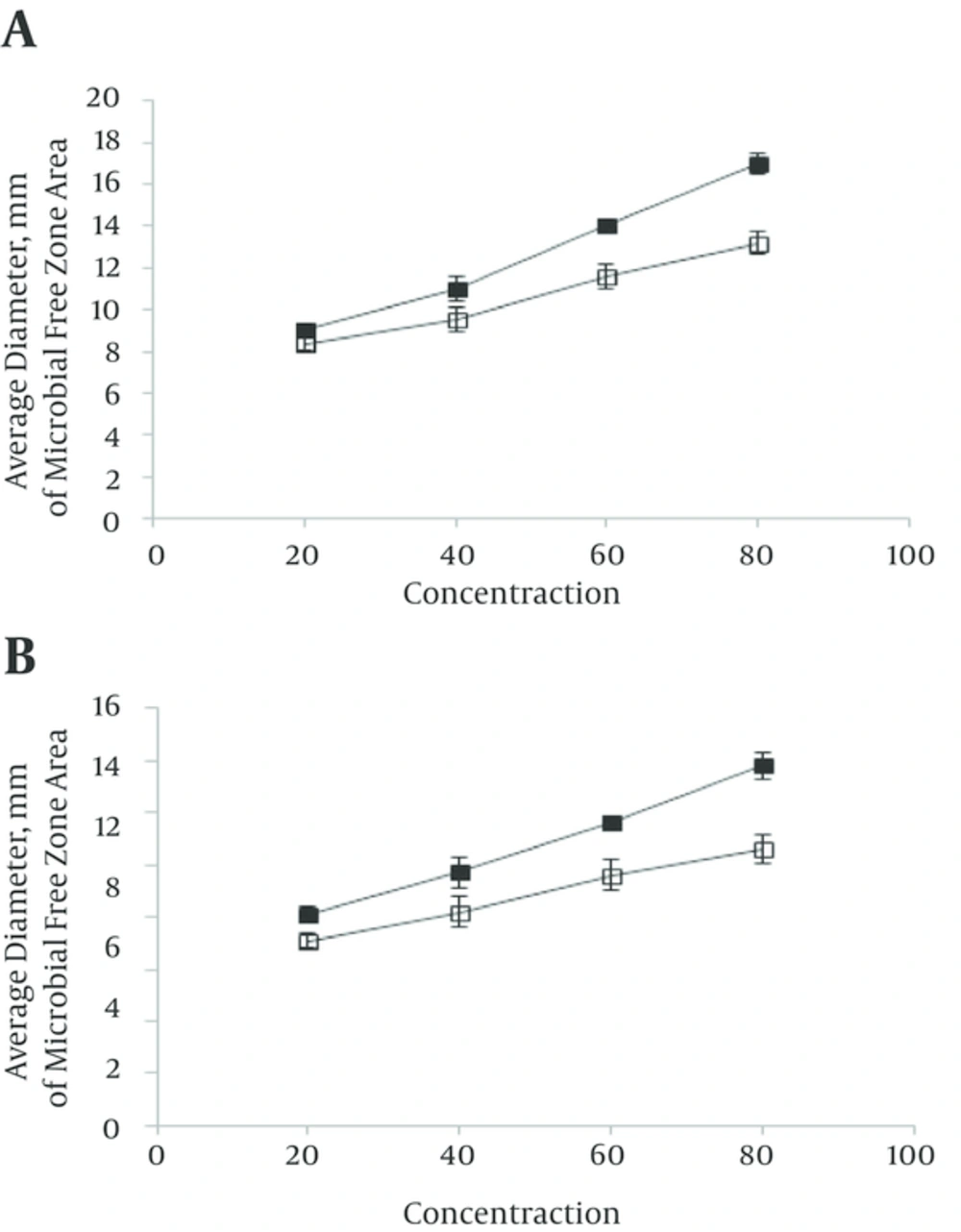
The results of antifungal effect’s mangrove leaf ethanolic extract in screening antimicrobial activity method were showed that mangrove leaf extracted in screening antimicrobial activity method in 2000 μg/mL, inhibit A. citri and P. digitatum growth (Figure 1). The results of antifungal activity of mangrove leaf aqueous extract in screening antimicrobial activity method were showed that the mangrove leaf extract in 2000 μg/mL, inhibit P. digitatum growth. However, 2,000 μg/mL concentration aqueous extracts, had no significant antifungal effect on A. citri and it is not able to prevent the growth of fungi on culture (Figure 1 and Table 1). The results showed that dry weight of ethanolic extract 4% was higher than the aqueous extract. The result shows that the mangrove leaf ethanolic extracts had inhibition effect on both A. citri and P. digitatum (in 20, 40, 60 and 80 mg/mL) but the mangrove leaf aqueous extract had inhibition effect on P. digitatum (in 20, 40, 60 and 80 mg/mL) and A. citri (in 40, 60 and 80 mg/mL) (Figure 2). The result shows that MIC of A. marina leaves ethanolic extract for A. citri and P. digitatum was 16 and 8 mg/mL respectively. The results show that MIC of aqueous extract of A. marina leaves for A. citri was 64 mg/mL and for P. digitatum was 16 mg/mL (Table 2). The results showed that MFC of aqueous extract of A. marina leaves for A. citri was 128 mg/mL and for P. digitatum was 32 mg/mL. The results showed that MFC of ethanolic extract of A. marina leaves for A. citri was 32 mg/mL and for P. digitatum was 16 mg/mL (Table 3).
| Extract (Avicenna marina) | Resulta |
|---|---|
| Ethanolic extract of A. citri | S |
| Ethanolic extract of P. digitatum | S |
| Aqueous extract of A. citri | R |
| Aqueous extract of P. digitatum | S |
aR, resistant; S, sensitive.
| Fungi Species | Concentration, mg/mLa | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | |
| Ethanolic extract of A. citri | - | - | - | - | + | + | + | + | + |
| Ethanolic extract of P. digitatum | - | - | - | + | + | + | + | + | + |
| Aqueous extract of A. citri | - | - | - | - | - | - | + | + | + |
| Aqueous extract of P. digitatum | - | - | - | - | + | + | + | + | + |
a+, positive inhibition; -, negative inhibition; n = 3.
| Fungi Species | Concentration, mg/mLa | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | |
| Ethanolic extract of A. citri | - | - | - | - | - | + | + | + | + |
| Ethanolic extract of P. digitatum | - | - | - | - | + | + | + | + | + |
| Aqueous extract of A. citri | - | - | - | - | - | - | - | + | + |
| Aqueous extract of P. digitatum | - | - | - | - | - | + | + | + | + |
a+, positive inhibition; -: negative inhibition; n = 3.
A, Control ethanolic extract of P. digitatum; B, antimicrobial effects of 2,000 μg/mL ethanolic extract of A. marina leaves; C, control ethanolic extract of A. citri; D, antimicrobial effects of 2,000 μg/mL ethanolic extract of A. marina leaves; E, control aqueous extract of A. citri; F, antimicrobial effects of 2,000 μg/mL aqueous extract of A. marina leaves.
5. Discussion
The result shows that the mangrove leaf ethanolic extracts had inhibition effect on both A. citri and P. digitatum (in 20, 40, 60 and 80 mg/mL) but the mangrove leaf aqueous extract had inhibition effect on P. digitatum (in 20, 40, 60 and 80 mg/mL) and A. citri (in 40, 60 and 80 mg/mL) (Figure 2). The results indicate that the ethanolic and aqueous extract of A. marina leaves had been effective on P. digitatum and has the least impact on A. citri.
It is important to study scientifically plants that have been used in traditional medicines to determine potential sources of novel antimicrobial compounds [12]. Plant based antimicrobial compounds have enormous therapeutically potential as they can prepared the purpose, without any side effects that are often associated with synthetic antimicrobials. Plants are used as important source for traditional medications [13]. Plants are rich in wide variety of phytochemicals like tannins, terpenoids, alkaloids, flavonoids, antimicrobial peptides, etc. that have been found to have antimicrobial activities [14-16]. The use of plant extracts in the treatment of diseases caused by various bacteria, viruses and fungi were reported. Antifungal properties of plants extracts are widely recognized [17]. Also, ethanolic extract compared to the aqueous extract was more effective. These results are similar with the findings of Khafagi et al. [18] on mangrove species. Ravikumar et al. [19] investigated the antibacterial effects of A. marina and reported that the extracted by the solvent methanol, ethanol and water had the highest antibacterial activity. In Ravikumar et al. study [19] the plant extracts were showed more inhibition effect on Gram positive bacteria (Staphylococcus aureus) than Gram negative bacteria (Pseudomonas aeruginosa, E. coli, Enterobacter spp. and Klebsiella pneumoniae). Antifungal metabolites of mangrove plant leaves include alkaloids, flavonoids and related compounds, fatty acids, oxygen heterocyclics, proanthocyanidins, quinones, stilbenes, terpenoids and triterpenoid, saponins [4]. The latex showed no inhibition effect against bacteria and yeast but had inhibition effect against some fungi. The leaves were a rich source of a different class of terpenoids and stilbenes, which inhibited histamine release from rat mast cells and were active against Bacillus and Staphylococcus [4]. Xanthone is an active substance in these plants. These compounds have toxicological characteristics, such as, anti-tumor, anti-inflammatory and anti-fungal activity [4]. The result shows that MIC of A. marina leaves ethanolic extract for A. citri and P. digitatum was 16 and 8 mg/mL respectively. The results show that MIC of aqueous extract of A. marina leaves for A. citri was 64 mg/mL and for P. digitatum was 16 mg/mL. Antifungal potential of seven methanolic mangroves bark extract was assayed by disk diffusion method against pathogenic fungi A. alternata and Fusarium moniliforme. Among these, methanolic extracts of Agiceras corniculatum showed maximum inhibition (56%) against A. alternata and Bruguiera gymnorrhiza exhibited maximum inhibition (57%) against F. moniliforme [20]. The antibacterial effects of A. marina, Ceriops decandra and Bruguiera cylindrica mangrove leaves were tested against antibiotic-resistant pathogens (ARB) including. S. aureus, Streptococcus pneumoniae, K. pneumoniae, P. aeruginosa and eye pathogens viz. E. coli, Proteus, Acinetobacter and Staphylococcus epidermidis. Most of the plant extracts showed promising antibacterial against both the bacterial groups [21]. The results show that MFC of aqueous extract of A. marina leaves for A. citri was 128 mg/mL and for P. digitatum was 32 mg/mL. The results show that MFC of ethanolic extract of A. marina leaves for A. citri was 32 mg/mL and for P. digitatum was 16 mg/mL (Table 3). The results indicated that ethanolic and aqueous extract of A. marina leaves mostly had been effective on P. digitatum and has the least impact on A. citri. Different levels of mangrove extract have been used to consider its antimicrobial effect. Fatty acids are widely occurring compounds in natural fats and dietary oils, and they are known to have antibacterial and antifungal properties [20, 22]. Fungal contamination of food products is a main problem in developing countries and it leads to a decline in quality and foodstuffs quantity. Some plant or spice extract can be used as food preservatives due to their strong antimicrobial activity. The results of this study showed that the mangrove leaves extract show antifungal properties justify their traditional use as medicinal plants. In conclusion, A. marina plants may have potential medicinal importance, and could suggest that A. marina leaf extract in vitro, have considerable antimicrobial ability over the studied strains. In addition, more studies are being done in situ, to identify the effective dose of the extract on the microorganisms and finally introduce the extract as a natural and novel antimicrobial compound.