1. Background
Ischemic brain damage is a major cause of adult disability. Forebrain ischemia, occurring during cardiorespiratory arrest in patients or experimentally in animals, induces selective and delayed neuronal cell death [1]. Forebrain ischemia induces complete interruption of blood flow, producing inadequate delivery of oxygen to brain tissue and leading to a decrease in glucose utilization and adenosine triphosphate (ATP). Oxidative stress is one of the primary factors that exacerbate damage by cerebral ischemia [2]. Several components of reactive oxygen species (superoxide, hydroxyl radical, hydrogen peroxide and peroxynitrite radical) that are generated after ischemia-reperfusion (IR) injury play an important role in neuronal loss after cerebral ischemia [3]. Superoxide and hydroxyl radicals are potent in producing destruction of the membrane by inducing lipid peroxidation [4]. The brain is particularly vulnerable to oxidative stress injury because of its high rate of oxidative metabolic activity, intense production of reactive oxygen species metabolites and high content of polyunsaturated fatty acids, relatively low antioxidants capacity, low repair mechanism activity and non-replicating nature of its neuronal cells [5]. During IR, tissues are subjected to the destructive proinflammatory cytokines and reactive oxygen species released by inflammatory cells leading to inflammatory injury and cell apoptosis. IR in one organ also affects the secondary organs, including liver, heart [6], kidney [7], lung [8] and even causes multiple organ failure (MOF), a common cause of mortality [9]. The concept of reperfusion injury has been a subject of debate for the past three decades, in which some investigators believe that all injury develops during the ischemic period whereas others argue that blood reflow extends tissue injury due to the release of oxygen-derived free radicals, dysregulation of intracellular and mitochondrial calcium, microvascular dysfunction leading to incomplete return of blood flow to areas of microcirculation, an overzealous inflammatory reaction involving influx of various populations of immune cells and delayed cell death due to apoptosis [10]. The phenomenon occurs in a wide range of situations including trauma, vascular reflow after contraction, percutaneous transluminal coronary angioplasty, thrombolysis treatment, organ transplantation and hypovolemic shock with resuscitation [11].
Herb medicines had traditionally played a major role in the management of human health and are still playing an active role in the health care in many countries. It has been suggested that for some herbs it is the natural antioxidants they contain conferred their biological activities [12]. Since during the IR injuries, reactive oxygen species (ROS) play the main role, using the herbs with antioxidative properties can be helpful in ameliorating the injuries. Salvia spp. has long used in Asian countries for clinical treatment of various microcirculatory disturbance-related diseases. This herbal drug contains many active water-soluble compounds which have ability to scavenge peroxides and are able to inhibit the expression of adhesion molecules in vascular endothelium and leukocytes [11]. Previous studies about anti-ischemic effects of Salvia miltiorrhiza in brain and liver of rats revealed that it can scavenge the free radicals, also can occlude calcium ion canals in hepatocytes and prevent hepatocytic injuries following ischemia reperfusion injuries [13, 14]. This plant also scavenges the free radicals producing in mitochondrial membrane of the myocardium following ischemia reperfusion injuries [15].
2. Objectives
According to antioxidative effects of different species of salvia genus, we decided to investigate the antioxidaive effect of Salvia rhytidia extract on histomorphometric changes of cerebral cortex and hippocampus following ischemia-reperfusion injuries. In the present study the extract of S. rhytidia, an endemic plant from south eastern of Iran, was used to prevent IR injuries induced in cerebral cortex and hippocampus of rats.
3. Materials and Methods
3.1. Animals
This cross-sectional study was performed on 35 male Wistar rats between 250 - 300 g. All animals were housed in an air-conditioned room (12 light/dark cycles with a temperature of 21ºC and relative humidity of 50%). The animals were fed commercial rodent pellet food and water. All the procedures were conducted in accordance with the European community guidelines for laboratory animals.
3.2. Experimental Groups and Surgical Procedures
Group A: Control; group B: No drug administration, 10 minutes ischemia, 24 hours reperfusion; group C: Manipulation of left common carotid and left vertebral arteries without ischemia, administration of normal saline, sacrificed after 24 hours; group D: 10 minutes ischemia, administration of S. rhytidia 2 hours after ischemia, sacrificed after 24 hours; group E: 10 minutes ischemia, administration of silymarin 2 hours after ischemia, sacrificed after 24 hours; group F: Administration of S. rhytidia, 72, 48, 24, 0 hours before ischemia, 10 minutes ischemia, 24 hours reperfusion; group G: Administration of silymarin, 72, 48, 24, 0 hours before ischemia, 10 minutes ischemia, 24 hours reperfusion.
The operation was performed under general anesthesia [40 mg/kg ketamine + 4 mg/kg xylazine intra muscular (i.m.)]. Following surgical preparations, left common carotid and left vertebral arteries were exposed through ventral midline cervical incision, separated from the vagosympathetic trunks and occluded for 10 minutes by Rumel tourniquet at the same time. After 10 minutes both of them were opened and the skin incision was closed with silk sutures, the animals were transferred to their cages.
3.3. Preparations and Administration of S. rhytidia Extract and Silymarin
S. rhytidia extract was taken from fresh aerial parts of the plants. The leaves were first made into a crude extract by immersion of the material in an aqueous solvent (70% ethanol) at room temperature for a sufficient time, typically 48 hours for all the soluble phytochemicals to dissolve. The solution was then concentrated using a climbing film evaporator or in a finishing still under a vacuum and dried in either a vacuum oven or by lyophilisation (freeze drying). The resulting compound was milled using 44 mesh into a powder resulting in an extract of dried leaf material of S. rhytidia. In group D, S. rhytidia extract (3.2 mg/kg) and in group E, silymarin (50 mg/kg) was administered orally 2 hours after operation. In group F the same dose of S. rhytidia and in group G, the same dose of silymarin was administered 72, 48, 24 and 0 hours before operation.
3.4. Sampling
At the end of the experiment, rats were deeply anesthetized by diethyl ether, the brains were dissected and cerebrums were removed and left hemispheres were sectioned and inserted in buffer formalin 10% for 24 hours.
3.5. Histological Study for Measuring Thickness of the Cerebral Cortex and Hippocampal Regions
The formaldehyde-fixed samples were dehydrated, cleared and embedded in paraffin. Sections of 5 µm thickness were cut, mounted and stained with hematoxyline-eosine method for histological evaluation. The thickness of the cerebral cortex and hippocampal regions was measured using a micrometric lens.
3.6. Statistical Analysis
The data were analyzed using SPSS-16, one way ANOVA, and Duncan as post hoc test and the level of significance was considered P < 0.05. The data presented in mean ± SEM.
4. Results
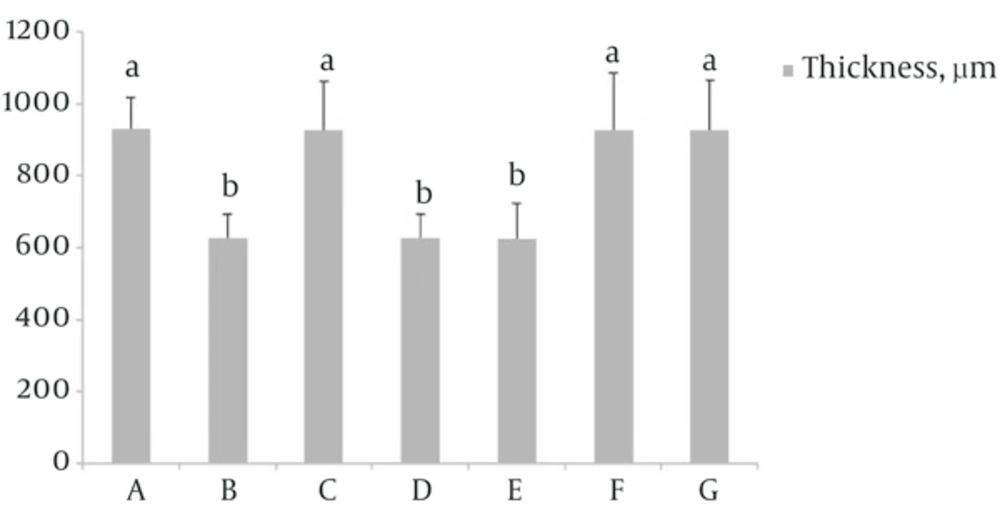
4.1. Effect of S. rhytidia Extract on Thickness of the Cerebral Cortex
Results showed that there was a significant decrease in thickness of the cerebral cortex in groups B, D and E compared to group A (P < 0.05). In group F which received S. rhytidia extract (3.2 mg/kg) and group G that received silymarin (50 mg/kg) before operation the cerebral cortex thickness did not significantly decrease compared to group A. The thickness in group C, partially was similar to group A (Figure 1).
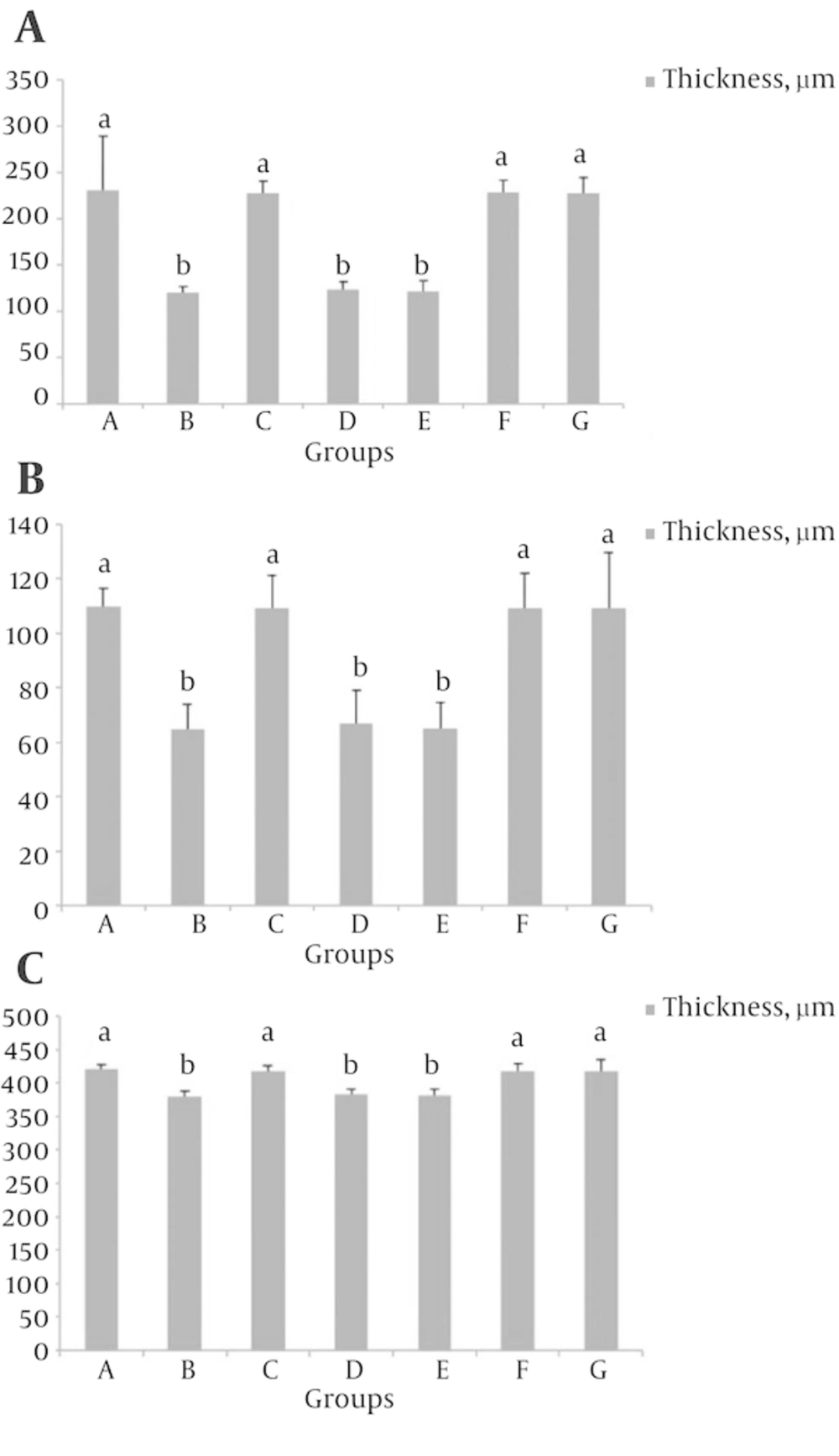
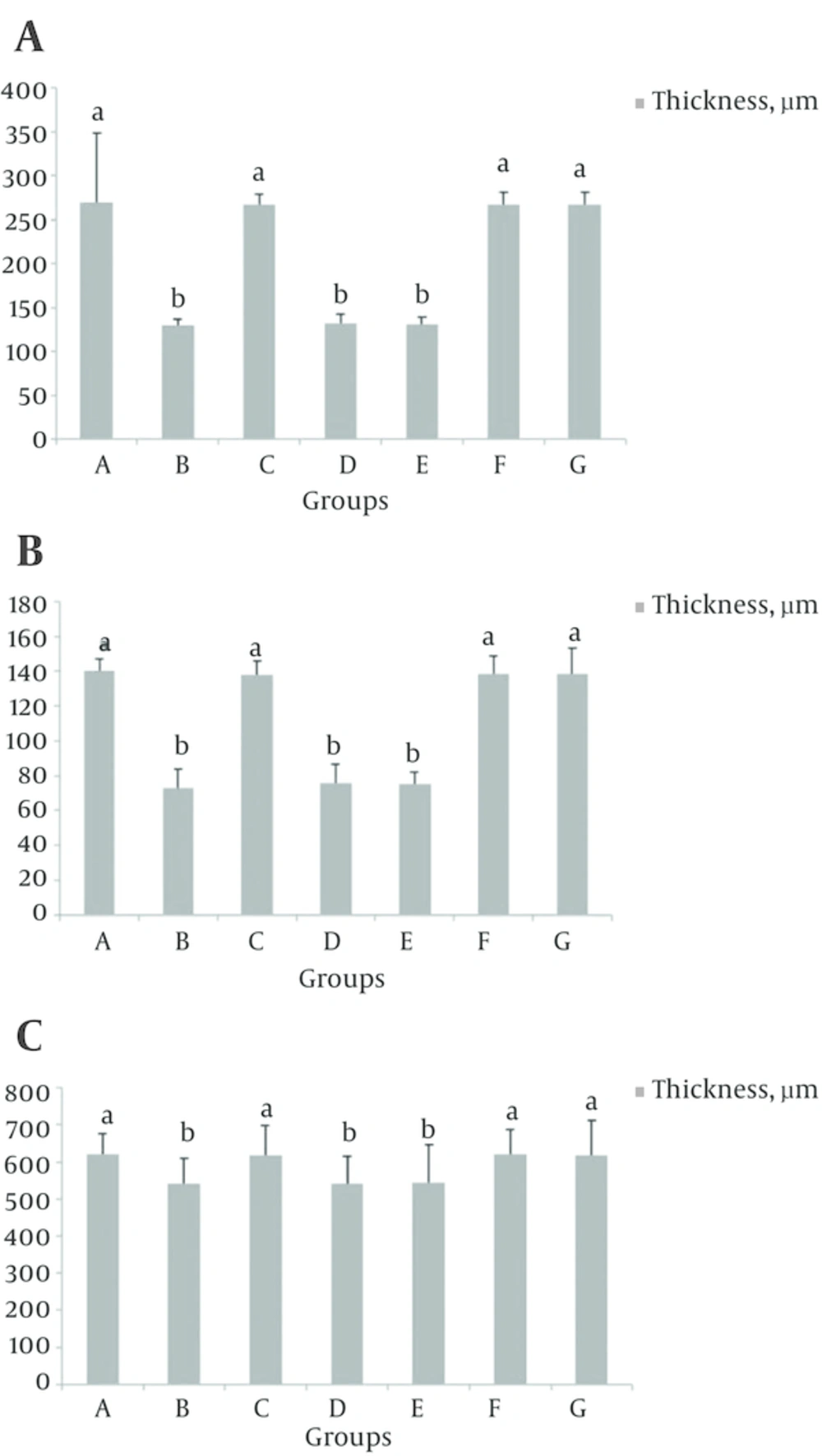
4.2. Effect of S. rhytidia Extract on Thickness of Different Zones of the Hippocampus (CA1, CA2, CA3 and Dentate Gyrus)
In groups B, D and E, the thickness of the CA1 and CA2 regions decreased compared to group A (P < 0.05), (Figure 2). While the thickness of the all hippocampal regions in groups F, G, the CA3 and dentate gyrus regions in groups B, D and E, did not significantly decrease compared to group A and the results in groups C and A, partially was similar to each other (Figures 3 - 5).
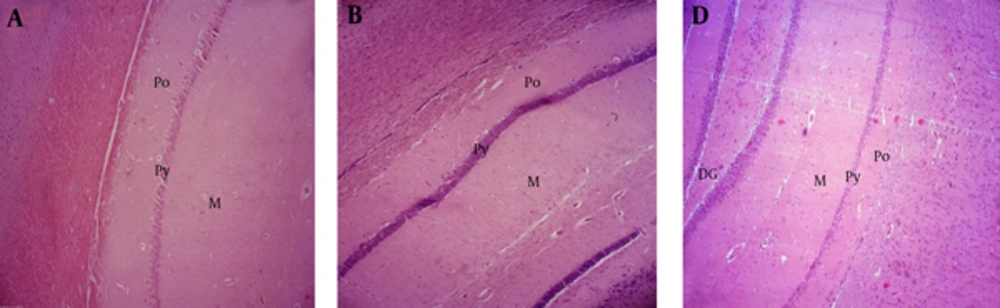
The results show that the thickness of the polymorphic (Po), pyramidal (Py) and molecular (M) layers of the CA1 zone, did not significantly decrease in ischemic groups that received S. rhytidia extract and silymarin 72, 48, 24 and 0 hours before ischemia (B), compared to control (A). The thickness of these layers significantly decreased in ischemic group without receiving drug and in groups that received S. rhytidia extract and silymarin, 2 hours after ischemia (C), compared to control (A).
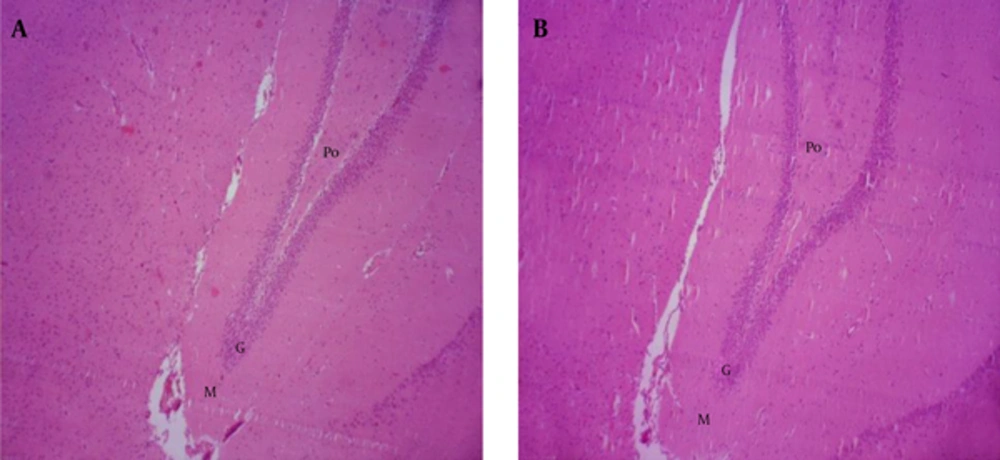
The results show that the thickness of the polymorphic (Po), granular (G) and molecular (Mo) layers of the dentate gyrus, did not significantly decrease in ischemic group without receiving drug and in groups that received S. rhytidia extract and silymarin 2 hours after ischemia (B), compared to control (A).
The different letters represent significant statistical difference between groups (P < 0.05). The results show that the thickness of the polymorphic layer in groups B, D and E significantly decreased compared to control (A), while the thickness of this layer did not significantly decrease in groups C, F and G compared to control.
The different letters represent significant statistical difference between groups (P < 0.05). The results show that the thickness of the pyramidal layer in groups B, D and E, significantly decreased compared to control (A), while the thickness of this layer did not significantly decrease in groups C, F and G compared to control.
The different letters represent significant statistical difference between groups (P < 0.05). The results show that the thickness of the molecular layer in groups B, D and E, significantly decreased compared to control (A), while the thickness of this layer did not significantly decrease in groups C, F and G compared to control.
The different letters represent significant statistical difference between groups (P < 0.05). The results show that the thickness of the polymorphic layer in groups B, D and E, significantly decreased compared to control (A), while the thickness of this layer did not significantly decrease in groups C, F and G compared to control.
The different letters represent significant statistical difference between groups (P < 0.05). The results show that the thickness of the pyramidal layer in groups B, D and E, significantly decreased compared to control (A), while the thickness of this layer did not significantly decrease in groups C, F and G compared to control.
The different letters represent significant statistical difference between groups (P < 0.05). The results show that the thickness of the pyramidal layer in groups B, D and E, significantly decreased compared to control (A), while the thickness of this layer did not significantly decrease in groups C, F and G compared to control.
5. Discussion
Results of our study revealed that 10 minutes ischemia following by 24 hours reperfusion in forebrain, significantly decreased the thickness of cerebral cortex and all layers of CA1 and CA2 regions of the hippocampus in groups B, D and E compared to group A. While in groups F which received S. rhytidia extract (3.2 mg/kg), and group G that received silymarin (50 mg/kg), 0, 24, 48 and 72 hours before ischemia, no statistical decrease observed in the thickness of the cerebral cortex and all regions of the hippocampus compared to control. Our study also demonstrated that ischemia-reperfusion did not significantly effect on thickness of the CA3 and dentate gyrus of the hippocampus compared to control.
Cerebral ischemia may be the result of cardiac arrest and/ or secondary focal ischemia following stroke or brain hemorrhage [16-18] Ischemic injury to neurons is primarily due to the interruption of blood flow, lack of oxygenation, drop of ATP and subsequent re-oxygenation of the brain ischemia-reperfusion [19]. Oxidative stress caused by ROS, is known to cause the oxidation of biomolecules leading to cellular damage. Increased lipid peroxidation (LPx) is the major consequence associated with oxidative stress [20].
It has already been a wide range of activities reported for the genus Salvia, which may be relevant for CNS disorders. One of them include antioxidant [21]. The essential oil of the plant, has been tested for its memory enhancing effect [22]. Generally the antioxidant effects of Salvia extracts have often been attributed to phenolic and monoterpenic compounds [23]. Rosmarinic acid is the predominant phenolic compound in Salvia and its effects is attributed to the compound’s antioxidant properties acting as scavenger of reactive O2 species [24]. The antioxidant activity of methanolic extracts of Salvia officinalis is comparable to ascorbic acid. Earlier studies attributed the antioxidant activity of S. officinalis to the presence of polyphenolic compounds [25, 26]. Studies about anti-ischemic effect of aqueous extracts of S. leriifolia leaf and seed in rat hippocampus show that neural cell injury in all regions of the hippocampus was reduced following pretreatment of animals with aqueous seed extract [27]. The results of another investigations indicated that pretreatment with aqueous and alcoholic extracts of S. leriifolia root significantly reduced the elevated concentration of lipid peroxides in rat hippocampus and muscle following ischemia-reperfusion injuries because of its antioxidant properties [27, 28].
Pyramidal neurons in the hippocampal CA1 region are particularly vulnerable to ischemia injury [29].
Results of a study revealed that ischemic injury increased apoptotic neuronal cell death in the hippocampal CA1 region, impaired short-term memory and decreased cGMP level. cGMP plays critical roles in modulating brain functions including neurogenesis and physiological and pathological apoptosis [30]. Another study indicated that increase of potassium was responsible for the decreased excitability in CA1 neurons after severe ischemia and involved in post ischemic cell death in hippocampus [31]. Previous studies also have been shown that neurons in the CA3 and dentate gyrus subfields of the hippocampus are resistant to short period of ischemia which is usually lethal to pyramidal neurons in hippocampal CA1 subfields [32].
Glutamate is the major excitatory transmitter in CNS. Many studies have proved that extracellular glutamate in the brain is accumulated after hypoxia-ischemic insult, which serves as excitotoxin and participates in the induction of delayed neuronal death after brain ischemia [33, 34]. Glial glutamate transporter1 (GLT-1) is a kind of predominant protein which removes glutamates from the extracellular space and maintains the extracellular glutamate below neurotoxic level in the brain [35]. Bruhn et al. observed a progressive post ischemic increase of GLT-1 expression in CA3 after mild brain ischemia for 10 minutes [36]. In animal ischemia-reperfusion model, Salvia prevented myocardial infarction injury, decreased the lipid peroxidation, enhanced antioxidant enzyme activities [12, 37-39] and scavenged free radicals [11, 12, 38, 40, 41].
According to results of this study, it can be concluded that pretreatment with alcoholic extract of S. rhytidia has potent antioxidant properties similar to silymarin, through which can reverse the IR injuries in the brain of rats.