1. Introduction
Nocardia spp. are related to the group of microorganisms known as the aerobic actinomycetes [1, 2]. These bacteria are beaded branching filaments, Gram-positive and partially acid-fast bacilli [1-4]. Nocardia infections can cause pulmonary disease in both immune-compromised and healthy individuals [4]. Nocardia infections are initiated by inhalation of the organisms or through traumatic(such as primary cutaneous Nocardial infection) [5, 6]. Nocardia species are found as saprophytic components in natural habitats such as soil, dust, fresh and saltwater or the surfaces of plants, so the presence of these agents in laboratory or respiratory samples may represents contamination or acquisition of infection from their natural environment that diagnosed with clinical signs, radiological finding and especially culture of Nocardia [1]. So far, Nocardia spp. has been isolated of soil and by culture media such as paraffin bait method, humic acid vitamin B agar, paraffin agar, sucrose‐gradient centrifugation, Lowenstein-Jenson, gelatin agar (GA), conventional media such as blood agar (BA) and urea agar [7-11]. Nocardia growth is slowly on non-selective culture media, and has the ability to grow in a wide temperature range. Isolation of Nocardia is difficult due to its slow growth which can be covered by the other fast-growing microorganisms. This study was performed to compare the several culture medium for choose more suitable medium for the isolation of the genus Nocardia from soil samples. Therefore, to achieve this aim was investigated the culture medium containing humic acid vitamin B agar, paraffin baiting method, paraffin agar and Sabouraud dextrose agar with cycloheximide.
2. Methods
A total of 400 soil samples were collected from February 2011 to September 2014 in different regions of Iran (Table 1) with approximately 4 cm deep. The samples were transferred to the laboratory immediately. In this study, for isolation of Nocardia from soil samples was used of 4 medium such as Humic acid vitamin B agar (HV agar), carbon free broth carbon-free broth (CFB) containing paraffin rods, paraffin agar (PA) and Sabouraud dextrose agar (SDA) whit cycloheximide. The composition of the HA agar is including 1.0 g Humic acid dissolved in 10 mL of 0.2 N NaOH, 0.5 g Na2HPO4 , 0.5 g , 1.71 g KCI , 0.05 g MgSO4.7H2O , 0.02 g CaCO3, 0.01 g FeSO4.7H2O, B-vitamins , 50.0 mg cycloheximide, 18.0 g Agar, 1.0 lit distilled water (7).
| Source of Soil Samples | The Number of Soil Samples Collected | The Number of Positive Samples of Soil | The Number of Nocardia Isolates in Each Culture Medium | |||
|---|---|---|---|---|---|---|
| Paraffin Bait Technique | HV Agar | PA | SDA whit Cycloheximide | |||
| North provinces | ||||||
| Gilan | 28 | 9 | 8 | 6 | 3 | 6 |
| Mazandaran | 40 | 16 | 13 | 3 | 5 | 1 |
| Golestan | 27 | 6 | 5 | 1 | 0 | 6 |
| Southern Province | ||||||
| Khuzestan | 13 | 0 | 0 | 0 | 0 | 0 |
| Hormozgan | 10 | 0 | 0 | 0 | 0 | 0 |
| Bushehr | 16 | 2 | 2 | 0 | 0 | 0 |
| Fars | 15 | 2 | 1 | 0 | 0 | 2 |
| Kerman | 8 | 1 | 1 | 0 | 0 | 1 |
| Eastern Province | ||||||
| North Khorasan | 15 | 1 | 0 | 0 | 0 | 1 |
| Razavi Khorasan | 34 | 3 | 0 | 0 | 0 | 3 |
| South Khorasan | 13 | 0 | 0 | 0 | 0 | 0 |
| Western Province | ||||||
| Ardabil | 7 | 0 | 0 | 0 | 0 | 0 |
| East Azerbaijan | 8 | 2 | 2 | 0 | 0 | 0 |
| Kurdistan | 7 | 0 | 0 | 0 | 0 | 0 |
| Kermanshah | 13 | 2 | 2 | 0 | 0 | 0 |
| Lorestan | 5 | 0 | 0 | 0 | 0 | 0 |
| Hamadan | 12 | 3 | 3 | 0 | 0 | 0 |
| Zanjan | 7 | 1 | 1 | 0 | 0 | 0 |
| Central provinces | ||||||
| Isfahan | 8 | 3 | 3 | 0 | 2 | 1 |
| Semnan | 15 | 3 | 2 | 0 | 0 | 2 |
| Qom | 13 | 0 | 1 | 0 | 0 | 0 |
| Yazd | 14 | 1 | 6 | 0 | 2 | 2 |
| Markazi Province (Arak) | 16 | 5 | 5 | 0 | 3 | 1 |
| Alborz | 20 | 6 | 0 | 0 | 0 | 0 |
| Tehran | 36 | 9 | 7 | 0 | 4 | 3 |
The Number of Soil Samples and Isolates of Nocardia from Iran Cities
Carbon-free broth medium (CFB) was used in paraffin baiting method, in which the sterile paraffin-coated glass placed into CFB [8, 11]. Sabouraud dextrose agar medium according to the manufacturer’s instructions was prepared. To preparation paraffin agar (PA) medium, Paraffin was added to Carbon-free agar (CFA) [11]. All of media were sterilized at 121°C for 15 minutes. The rate of 0.1 mL of suspensions each soil sample was placed and spread with a sterile loop simultaneously. About 1 mL of inoculate (soil sample) was added to a test tube containing 5 mLof sterile carbon-free broth and a paraffin-coated glass. Then these plates were incubated at 35°C and microbial on each medium was evaluated during a month. Nutrient agar (NA) medium and Sabouraud dextrose agar (SDA) were used to purify isolates. Colonies were grown in 35°C within 2 to 7 days. Identification of the genus Nocardia was based on Gram-positive, acid-fast and partially acid-fast staining, resistant to lysozyme and morphological tests such as size, shape and aerial hyphae [12-14].
3. Results
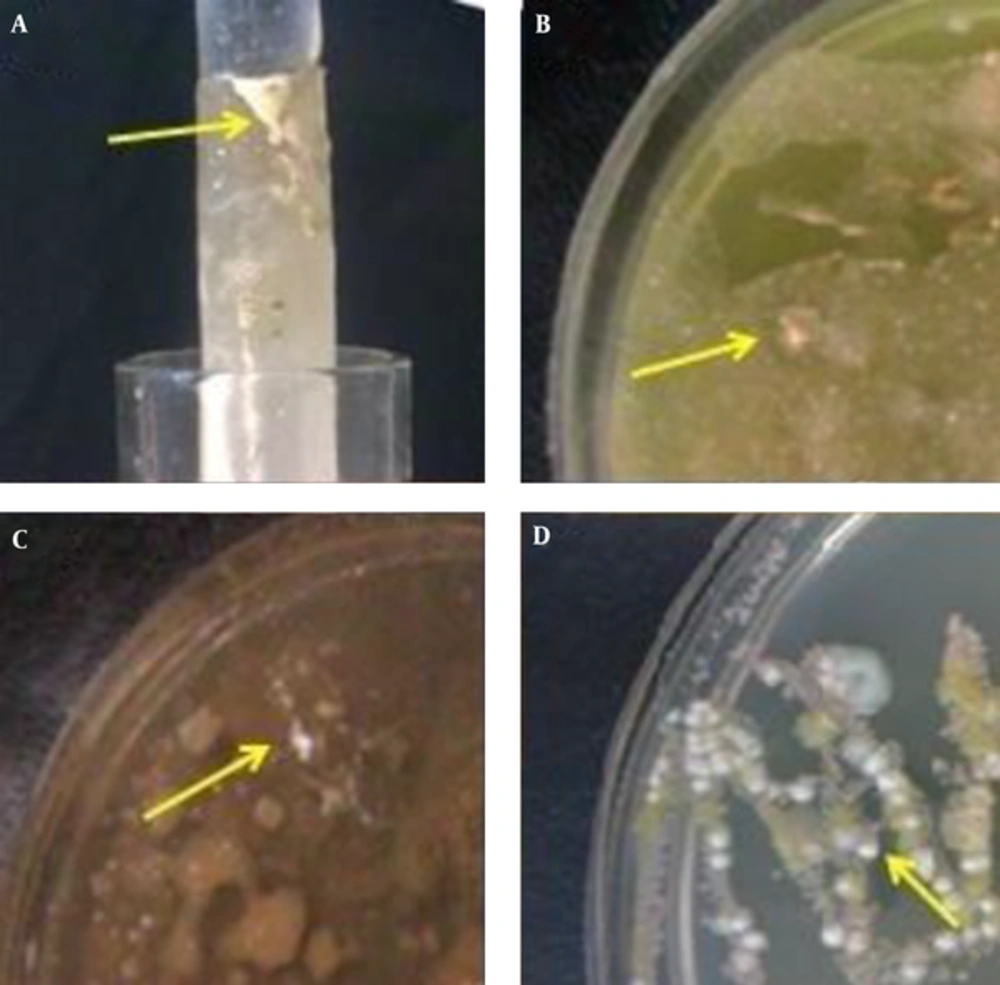
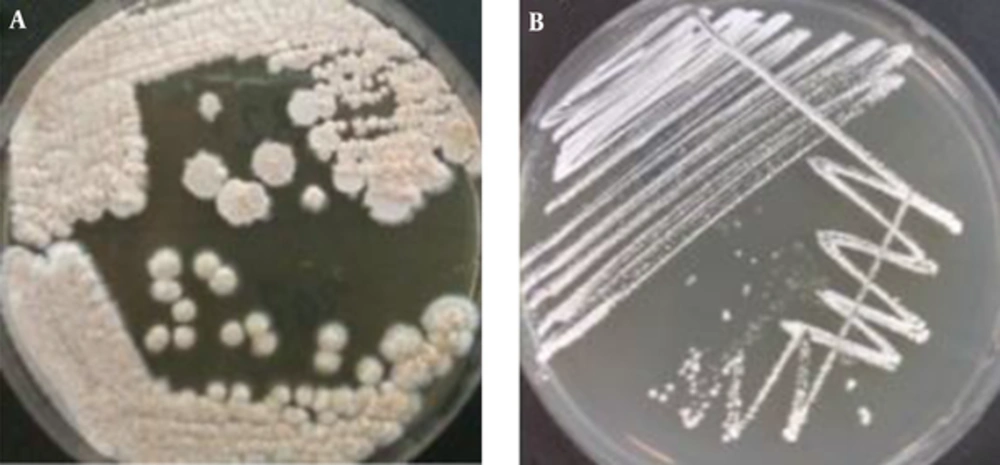
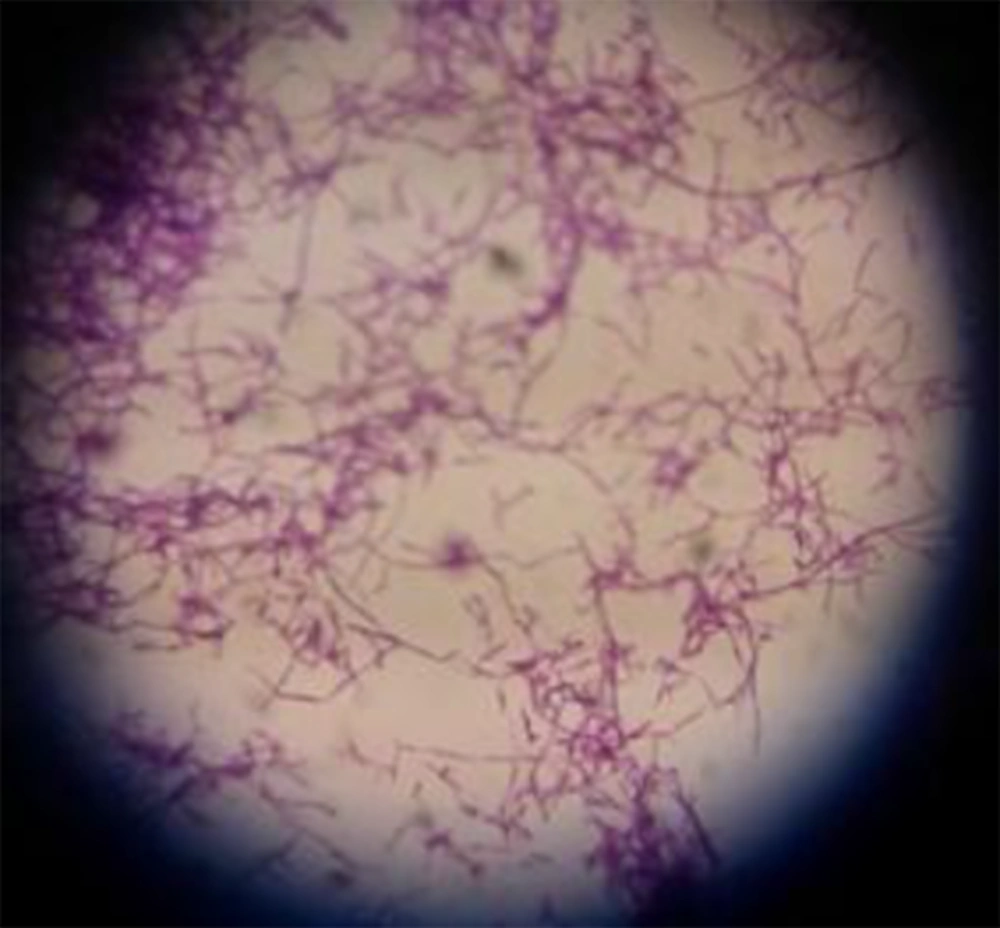
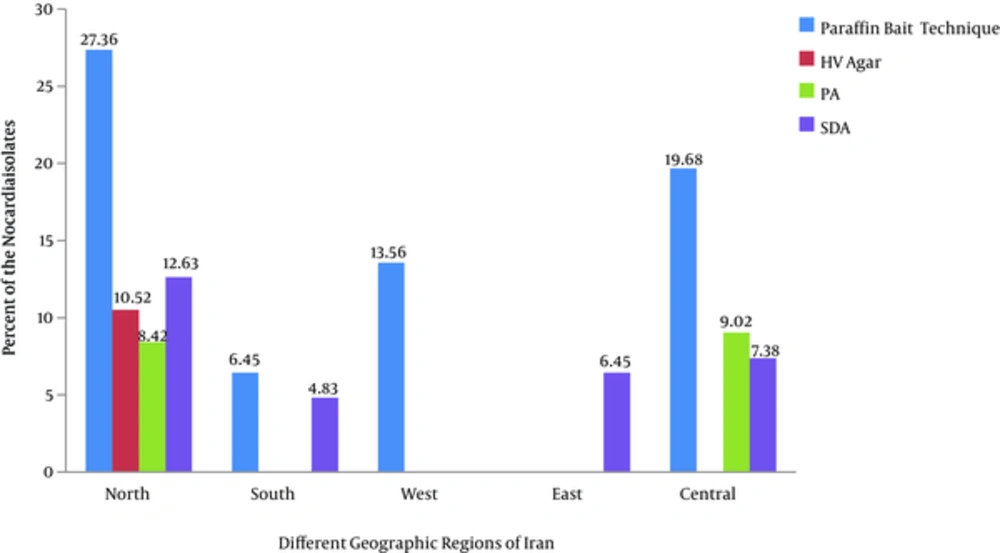
After inoculum of soil samples to each culture media (carbon-free broth containing paraffin rods, paraffin agar, humic acid vitamin B agar and Sabouraud dextrose agar whit cycloheximide separately and incubated at 35°C, Nocardia growth was observed within 1 to 4 weeks (Figure 1). The 119 Nocardia colonies were observed on the culture media in 75 soil samples. In our study, both nutrient agar (NA) and SDA (without cycloheximide) were used to obtain pure cultures of Nocardia isolates (Figure 2). Nocardia colonies have a variable appearance. Colony morphology of 3 - 10 days pure cultures was smooth and moist, plaster, star-shaped, chalking wrinkled whit the colors white, orange and pink, creamy (Figure 2). Nocardia isolates were Gram-positive, non-acid-fast, partially acid-fast (Figure 3) and resistant to lysozyme (Figure 4). A total of 400 collected soil samples, 62 strains of Nocardia (15.5%) were isolated by paraffin bait, 10 isolates (2.5%) by Humic acid vitamin B agar, 28 isolates using paraffin agar (7% ) and 19 isolates using the Sabouraud dextrose agar with cycloheximide (4.75%) (See Table 1). Growth of Nocardia spp. was observed on each of four media in 10 soil samples. Most Nocardia isolates were obtained from soil samples collected from Mazandaran (40%) (Figure 5).
4. Discussion
Nocardia is an aerobic, non-motile, partially acid-fast, rod-shaped and beaded branching filaments bacterium [15]. The genus Nocardia was isolated first by Edmond Nocard in 1888 from a case of bovine farcy. The genus of Nocardia is found commonly throughout the world in soil. Various studies have demonstrated that isolated from soil, plants, dust, decaying vegetation, and decaying fecal deposits from animals [8, 16]. Nocardia species cause a wide spectrum of diseases including pulmonary nocardiosis; cutaneous and subcutaneous nocardiosis [1, 17, 18]. In a study by DeCroos et al. ocular exposure to soil or plant matter was a common historical point in cases of Nocardia keratitis (48%) and scleritis (45%), respectively [5]. Nocardia produce the secondary metabolism such as antibiotics like tubelactomicin A. Therefore, isolation and identification of Nocardia species from natural habitat is important [1, 19]. The numbers of Nocardia species are increasing therefore accurate identification of Nocardia spp. is crucial to treatment of the Nocardial infections and also to identify new species of Nocardia [20, 21]. It is noteworthy that diagnosis through biochemical and phenotypic methods are not enough. It would be better that a molecular technique had been used. Isolation of microorganism is first step for the identification. Various methods include pretreatment techniques in combination with enrichment techniques and selective antimicrobial agents have been developed to isolation the actinomycetes genera from natural habitats [22]. Nocardia can grow on routine bacterial, fungal, or mycobacterial culture media [23]. Few studies have been carried out in the isolation of Nocardia from soil using various methods. Isolation these bacteria from a mixture samples can sometimes be facilitated by the use of suitable combination (In order to create the selective media). For example, isolation of Nocardia spp. is enhanced by the use of paraffin, while many microorganisms cannot be use of paraffin as the sole carbon source [8, 11]. In a study by Khan et al. in 1997 were isolated 41% of strains of the genus Nocardia using the paraffin bait of 102 soil samples [22, 24]. In a study conducted in 2012, HA agar allowed the growth of the largest numbers of actinomycetes colonies belonging to each genus of Streptomyces, Micromonospora, Microbispora, Streptosporangium, Nocardia, Dactylosporangium, Microtetraspora and Thermomonospora on the plate. HV agar was as the sole source of carbon and nitrogen was developed [7] According to studies by Hayakawa et al, HV agar may be recommended for greater growth of actinomycetes because of the activation of spore germination of actinomycetes is may be considered. In our study, the 10 colonies of Nocardia were recovered on HV agar media that less capable for isolation of these bacteria to compared with the other two (SDA and PA). In the present study, bacterial growth on the HV agar medium was observed at least three weeks to form small orange/ yellow colonies (see Figure 3B). In a study, paraffin agar medium was introduced an inexpensive and selective medium for isolation of Nocardia from clinical compared with modified thayer martin medium and Paraffin baiting techniques [25]. In current study, PA medium due to its fragile, oily surface and high pollution and also is not recommended for separation these bacteria (see Figure 3C). Nocardia growth on this medium was observed after the third weeks. Despite greater clarity and earlier appearance of Nocardia colonies on SDA medium (See Figure 3D) than the PA medium, the low numbers of colonies were isolated on this media. In a study conducted by Aghamirian et al. 96 aerobic actinomycetes were isolated from 300 the soil samples of Qazvin province on brain-heart infusion agar and Sabouraud dextrose agar (SDA) [26]. In these cases, white and cream-colored growth was observed on the paraffin-coated rod above the surface of the carbon-free broth (See Figure 3A). The growth of the bacteria was observed on the paraffin coated rods and SDA earlier (2 to 4 weeks and in some cases in the first week). Plaster and white colonies of Nocardia were seen more on the paraffin rods in paraffin baiting media. In studies conducted by Shawar et al. Gugnani et al. and Bafghi et al. the paraffin bait technique can also be used for the recovery of Nocardia from clinical samples [16, 27, 28]. In our study the paraffin baiting technique is a more appropriate than other methods to isolation Nocardia from soil. Colonies of Nocardia in this technique were detected better than other methods. The aim of this study was not to investigate the epidemiology, but we found that the most Nocardia species were isolated further on the northern edge (32.63%) of the Iran country with moderate climate. It seemed that Nocardia growth in these areas has increased under the influence of factors such as organic matter, temperature, moisture, soil pH, soil nutrient cycling, vegetation and climatic conditions also. So, Nocardia species is varied in different geographic locations. See the percent of isolation of Nocardia by each of the culture media in different geographic regions of Iran in Table 1. It is seems, some selective medium are better for the recovery of Nocardia. Isolation of Nocardia species is enhanced by the use of paraffin baiting, which relies on the selective ability of the organism to metabolize paraffin.
4.1. Conclusions
Paraffin baiting technique due to less cost and more detection of colonies similar to Nocardia is better than others methods as a routine laboratory diagnostic procedure.