1. Background
Cystic echinococcosis (CE), also known as hydatid cyst, is one of the main zoonosis infections, which is caused by Echinococcus granulosus at the larval stage (1). This disease is widespread in most countries of the world, including Australia, South America, Middle East, Eastern Europe, South Africa, and the Mediterranean region (2).
Three different treatment options to eliminate hydatid cysts, include surgery, chemotherapy, and percutaneous aspiration (3). Inoperable cases are treated using benzimidazole chemotherapy, which is recommended as the best alternative to surgical methods (4). In spite of its efficacy, chemotherapy poses different side effects, such as hepatotoxicity, severe leucopenia, thrombocytopenia, and alopecia (5). Furthermore, resistance to synthetic anthelmintics in treating cystic echinococcosis has made researchers look for other beneficial scolicidal agents, such as medicinal herbs, which pose less adverse effects (3, 6).
Oak is a plant that is related to the genus Quercus in the Fagaceae family. It grows in Asia Minor, Iran, and Greece and it is well-known for its medical properties all over the world (7, 8). Furthermore, Q. infectoria was once used as a natural remedy for curing various diseases and it is abundantly found in Zagros mountains in the west of Iran (7).
Several studies have investigated Q. infectoria and its derivative antibacterial (9), antifungal (10), antiviral (11), and anti-parasitic (12) effects. Analgesic quality of Q. infectoria’s methanol extract has also been confirmed previously (13).
Presence of polyphenolic compounds in crude extract of Q. infectoria engendered antioxidant, anti-inflammatory, antimicrobial, and anticancer qualities. These compounds include gallic acid and tannins, and some other flavonoid compounds, such as quercetin (12, 14).
2. Objectives
To the best of the author’s knowledge, there are no researches that have investigated the scolicidal effects of Q. infectoria. Hence, the present study aimed at investigating Q. infectoria Olivier extract’s in vitro effects on eliminating hydatid cysts.
3. Methods
3.1. Collecting Protoscoleces
In this experimental study, the protoscoleces were taken from livers of infected sheep, which were slaughtered at Urmia abattoir, Northwest of Iran. The hydatid fluid was transferred under aseptic conditions to a flask and left for 30 minutes in order to settle down the protoscoleces (15). Phosphate buffered saline (PBS) solution (pH = 7.2) was used to wash the protoscoleces for three times after removing the supernatant. The viability of the protoscoleces was confirmed from the motility features of protoscoleces, using an ordinary light microscope following staining with 0.1% eosin.
3.2. Collecting Plant Materials and Extraction
The oak plants were obtained from mountains of Sardasht (West Azerbaijan, Iran) on September, 2015. The obtained samples were identified and confirmed by the agriculture faculty of Urmia University (Urmia, Iran).
Some modification was done on the method described by Moazeni and Roozitalab (15) to prepare the methanolic extract of Q. infectoria. Briefly, a commercial electric blender was utilized to powder the dried plant. A small amount of the obtained powder (100 g) was added to 400 mL of pure methanol and gently mixed for one hour by a magnetic stirrer. The resulting solution was left intact for 24 hours at room temperature. After 24 hours, the solution was stirred again and filtered. Using a rotating evaporator, the solvent was removed. The material was left in semisolid form and it was freeze dried. It was stored at 4°C for further usage.
3.3. Scolicidal Effects of Quercus inferctoria
Quercus infectoria extract with three different concentrations of 10, 25, and 50 mg/mL were used with different exposure times (10, 20, 30, and 60 minutes) in order to study their efficacy in eliminating protoscoleces of hydatid cysts. Furthermore, 0.5 mL of protoscoleces (2 × 103/mL) solution was placed in test tubes. Then 0.5 mL of each concentration was added to each test tube (16). They were gently mixed and then left intact for 10, 20, 30, and 60 minutes at room temperature. At each interval, the upper phase of the mixture was taken by a pipette. This was done very carefully to avoid disruption of the protoscoleces. Staining was done by adding 2 mL of 0.1% eosin to the settled protoscoleces in the tube and it was gently mixed (15). The upper position of the remaining solution was discarded. The remaining protocoleces were smeared on a glass slide to be examined with a light microscope in order to investigate their viability. Dead protoscoleces’ percentage was obtained by counting a minimum of 450 (mostly more than 500) protoscoleces. Normal saline was selected for utilization as the control group and the experiments were performed in triplicates.
This study used 0.1% eosin (1 g eosin in 1000 mL of distilled water) in order to analyze protoscoleces viability. Fifteen minutes of exposure to eosin had no significant effect on the color of viable cells, yet it made the dead cells turn red due to absorbing eosin. By dividing the percentage of dead protoscoleces to their sum total, the mortality rate was measured (16).
3.4. Statistical Analysis
The SPSS software (version 19, Chicago) was the software used to perform the statistical analysis. Chi-square test was utilized to analyze the difference between test and control groups. Values less than 0.05 (P < 0.05) were considered significant.
4. Results
Results of the effectiveness of different concentrations of Q. infectoria extract as a scolicidal agent are summarized in Tables 1-3. It was observed that the Q. infectoria extract at all concentrations exhibited significant scolicidal effects in comparison with the control group (P < 0.05).
| Exposure Time (Min) | Experiments | Total | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| 10 | ||||
| Protoscoleces | 430 | 400 | 421 | 1251 |
| Dead protoscoleces | 105 | 90 | 94 | 289 |
| Mortality rate (%) | 25.41 | 22.5 | 22.32 | 23.1 |
| 20 | ||||
| Protoscoleces | 421 | 429 | 457 | 1307 |
| Dead protoscoleces | 178 | 167 | 162 | 507 |
| Mortality rate (%) | 42.28 | 38.92 | 35.44 | 38.7 |
| 30 | ||||
| Protoscoleces | 467 | 469 | 478 | 1414 |
| Dead protoscoleces | 282 | 283 | 279 | 844 |
| Mortality rate (%) | 60.38 | 60.34 | 58.36 | 59.6 |
| 60 | ||||
| Protoscoleces | 450 | 445 | 457 | 1352 |
| Dead protoscoleces | 328 | 321 | 333 | 982 |
| Mortality rate (%) | 72.8 | 72.1 | 72.8 | 72.6 |
| Control | ||||
| Protoscoleces | 624 | 502 | 521 | 1647 |
| Dead protoscoleces | 76 | 60 | 58 | 194 |
| Mortality rate (%) | 12.17 | 11.9 | 11.13 | 11.7 |
| Exposure Time (Min) | Experiments | Total | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| 10 | ||||
| Protoscoleces | 400 | 478 | 426 | 1304 |
| Dead protoscoleces | 202 | 210 | 203 | 615 |
| Mortality rate (%) | 50.5 | 43.93 | 47.65 | 47.16 |
| 20 | ||||
| Protoscoleces | 414 | 409 | 425 | 1248 |
| Dead protoscoleces | 232 | 231 | 225 | 688 |
| Mortality rate (%) | 56.03 | 56.4 | 52.9 | 55.1 |
| 30 | ||||
| Protoscoleces | 462 | 455 | 465 | 1382 |
| Dead protoscoleces | 35 | 37 | 29 | 101 |
| Mortality rate (%) | 75.7 | 81.3 | 62.3 | 73.08 |
| 60 | ||||
| Protoscoleces | 413 | 425 | 415 | 1253 |
| Dead protoscoleces | 392 | 377 | 391 | 1160 |
| Mortality rate (%) | 94.91 | 88.7 | 94.2 | 92.57 |
| Control | ||||
| Protoscoleces | 624 | 502 | 521 | 1647 |
| Dead protoscoleces | 76 | 60 | 58 | 194 |
| Mortality rate (%) | 12.17 | 11.9 | 11.13 | 11.7 |
| Exposure Time (Min) | Experiments | Total | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| 10 | ||||
| Protoscoleces | 475 | 471 | 482 | 1427 |
| Dead protoscoleces | 405 | 425 | 427 | 1257 |
| Mortality rate (%) | 85.26 | 90.23 | 88.58 | 88.08 |
| 20 | ||||
| Protoscoleces | 459 | 467 | 475 | 1383 |
| Dead protoscoleces | 459 | 467 | 475 | 1383 |
| Mortality rate (%) | 100 | 100 | 100 | 100 |
| Control | ||||
| Protoscoleces | 624 | 502 | 521 | 1647 |
| Dead protoscoleces | 76 | 60 | 58 | 194 |
| Mortality rate (%) | 12.17 | 11.9 | 11.13 | 11.7 |
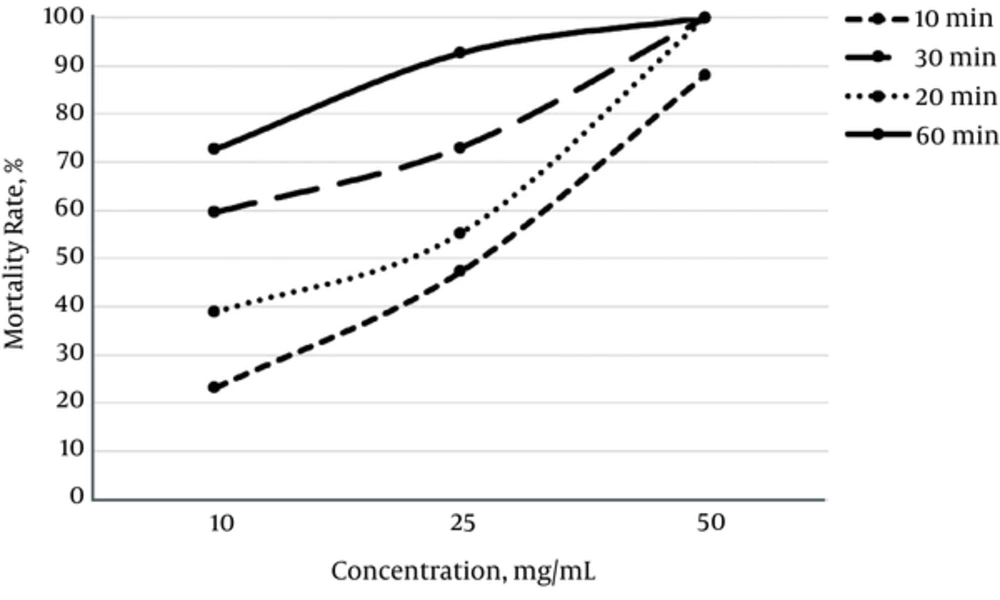
Furthermore, Q. infectoria extract at a concentration of 25 mg/mL killed 47.16%, 55.1%, 73.08%, and 92.57% of the protoscoleces after 10, 20, 30 and 60 minutes of application, respectively, while the mortality rate of protoscolices in the control group was 11.7%. In addition, all protoscoleces were killed after 20 minutes of exposure to 50 mg/mL of Q. infectoria extract (Figure 1). By increasing the exposure time with Q. infectoria extract at all concentrations, the mortality rate was significantly increased (P < 0.05) (Figure 2). Therefore, the results of the current study indicated that the methanolic extract of Q. infectoria has high scolicidal activity in vitro.
5. Discussion
In this study, the researchers investigated the potency of Q. infectoria extract on prtoscolices of hydatid cyst. Surgery is the most preferred method for removing E. granulous cysts in order to cure hydatid cysts (17). So far, different sclocidal agents were utilized to counteract the contents of hydatid cysts, yet no one proper strategy has been figured out to eradicate this disease (18, 19). Qualities, such as low cost, potency at low concentrations, lack of adverse effects, quick action, and non-toxicity are among the desired qualities for a good scolicidal agent (20).
Less adverse effects, low price, and high availability are qualities that made herbal extracts a good alternative for treating various diseases. There have been numerous studies on scolicidal effects of different plants, such as Nigella sativa (16), Allium sativum (21), Curcuma long, and Zingiber officinale (22), Salvadora persica (23), and Zataria multiflora (15) to use them in curing hydatid cysts.
Quercus infectoria has been widely used in traditional medicine for analgesic, CNS depressant, antidiabetic, anti-inflammatory, and antiparkinsonian purposes (24). In addition, Q. infectoria is considered a safe and nonpathogenic plant for cells of mammalian species (12).
Reports on the effects of this herbal extract delineate that Q. infectoria is composed of various components, which target several points in microorganism cells (25). As mentioned earlier, polyphenolic compounds like tannins and gallic acid, along with flavonoid compounds, such as quercetin, are among the most significant components of Q. infectoria (12, 14), and it is assumed that they are responsible for its biological efficacy. Studies has also revealed antiplasmodial, antileishmanial, and trypanocidal effects of flavonoid compounds (26, 27).
There haven’t been any studies investigating scolocidal qualities of Q. infectoria. This is the first study analyzing the effects of oak extract in vitro condition on hydatid cysts.
This study showed that Q. infectoria’s scolicidal activity is significant at concentrations of 50 mg/mL after 20 minutes of exposure, which leads to a 100% mortality rate. In other studies, Moazeni and Roozitalab (15) investigated the protoscolicidal activity of Zataria multiflora extract. They concluded that Zataria multiflora extract had 100% scolicidal effect at a concentration of 25 mg/mL after one minute of exposure, whereas the application of Garlic (Allium sativum) extract produced excellent results (100%) over killing of protoscolices at a concentration of 50 mg/mL after 10 minutes (21).
Quercus infectoria’s scolicidal effects at the concentration of 50 mg/mL after 20 minutes of exposure was similar to some other agents, such 95% ethyl alcohol (15 minutes) (28), 0.5% to 1% cetrimide (10 minutes) (29), 3% H2O2 (15 minutes) (30), and 20% hypertonic saline (15 minutes) (31). In the present study, the researchers observed a higher scolicidal effect (100%) with Q. infectoria extract at a higher concentration (50 mg/mL).
Furthermore, this research showed higher in vitro concentrations of Q. infectoria extract were toxic for protoscoleces of E. granulosus, which might be due to the presence of flavonoid compounds. In line with the current results, Kheirandish et al. (12), showed that high amounts of phenolic compounds extract of Q. infectoria might be the active compound responsible for anti-leishmanial activity.
In conclusion, the current findings demonstrated that Q. infectoria could be used as an effective scolicidal agent. Further investigations are required to determine the potential adverse effects of Q. infectoria and to confirm the scolicidal efficacy of this herbal medicine in vivo.