1. Background
Running is an excellent physical activity, which can improve well - being and general health (1). Moreover, it is among the most common weight - bearing exercises (2, 3). One common concern related to running is the possible long - term effect on the body. In fact, running considerably increases the amount of force on the ankle (4) and hip (5) joints, when compared with walking on a level surface.
Based on some studies, exercise is an effective nonpharmacological treatment for knee osteoarthritis (OA) (6). Although several studies noted the negative effects of intensive exercise (7, 8), most researchers concur that moderate - intensity exercise can be advantageous (7, 9). Nonetheless, in excessive running loading, adverse effects on the cartilage are reported (9, 10).
OA is described as a degenerative and slowly progressive disorder, during which gradual loss of articular cartilage occurs. OA shows a higher prevalence among females in comparison with males and is often generalized in females (7, 11). Currently, the cause of this variation is undetermined (7, 9), although gender differences in cartilage volume can potentially explain this difference (7). In a study by Ding et al., knee cartilage was more common in healthy male children in comparison with females, even after adjusting for other confounding factors. It was concluded that gender differences in knee OA in later stages of life might be attributed to differences in cartilage development (12).
In some studies, gender differences of similar magnitude were reported in tibial and femoral cartilage volume among young healthy subjects. After adjusting for height and body weight, these differences became insignificant (13). The observed discrepancies might be attributed to differences in subjects’ age, sample size, and type of the samples. In addition, as young subjects were commonly examined in previous studies, it is not clear whether gender differences are associated with a combination of development and loss or cartilage development (7).
Exercise - induced animal model is a more reliable model of OA in comparison with other models (14, 15). On the other hand, although several studies are conducted on the occurrence of OA in males or females, there is not enough information about the effect of a same exercise on histomorphometric and histopathologic changes in the knee joint both in males and females. Therefore, the current study aimed at comparing the histopathological changes of knee joint after six weeks of running on a treadmill with 18° inclination in adult male and female Wistar rats with the same age and body weight.
2. Methods
2.1. Animal Ethics
All procedures involving the experimental use of animals were approved by the Animal Ethics Committee, a branch of the Research Council of the Veterinary School in Shahid Bahonar University, Kerman Province, Iran.
2.2. Animals
Forty adult male and female Wistar rats (9-10 - week - old) with no significant difference in their body weight (250 - 265 g) were randomly divided into four equal groups. All rats were allowed a five - day adaptation period in a room with controlled conditions (temperature 22-25°C and humidity 60%-70%) before starting the experiment (16).
2.3. Exercise Protocol
Ten subjects of each gender were selected as male and female control groups, while running exercises were performed in the remained 20 male and female rats for six weeks on a motor - driven rodent treadmill (Model T510, DRI Co., Taoyuan, Taiwan), speed of 20 m/minute with inclination of 18° for 60 minutes each day and five days a week.
2.4. Histological Preparation
On the day 43, all control and runner rats were euthanized and the whole rat knee joints were taken and fixed in 10% buffered formaldehyde for 10 days. Decalcification in 10% formic acid solution was followed by embedding of the complete knee joints in this solution for 50 days. Five micrometer thickness serial sections of frontal whole left knee joints were prepared in order to obtain the thickest cartilage section. Obtained sections were stained with hematoxylin - eosin (H & E) method.
2.5. Histological and Histomorphometrical Techniques
Sections were evaluated using the Mankin scheme (17). Only the safranin - O staining was differentiated with H & E method. The histological evaluation system for OA was classified into four categories: Mankin score of 0: no OA; scores of 1-5: mild OA; scores of 6-10: moderate OA; and scores of 11-14: severe OA (18). In order to histological grading of synovial layer changes, the Krenn et al., method was used. Based on their results, 0 to 1 corresponded to no synovitis, 2 to 3 to a slight synovitis, 4 to 6 a moderate synovitis, and 7 to 9 a strong synovitis (19).
Histomorphometric analyses were performed on sections using the method described by Renner et al. Thickness from subchondral bone to articular surface was measured at the middle of lateral condyle. In each section, chondrocyte cells were counted within a 120000 µm2 area including both calcified layer and articular surface (15).
All right knee joints were opened and examined for gross knee articular surface evaluation.
Results were expressed as mean ± standard error (SE) with SPSS version 16. Statistical analysis was conducted using an independent samples t test and one - way ANOVA. Also, the Tukey test was used for post hoc analysis with significance level set at P < 0.05.
3. Results
3.1. Gross Evaluation of Knee Articular Cartilage
Cartilage surface in both male and female control groups appeared glossy and translucent, with a smooth surface. In contrast with them, the articular surfaces of four male runner rats were irregular at the end of the week 6. On the other hand, superficial cartilage irregularities in the femur condyles and trochlea were found in all female examples of runner group (Figure 1). The depth and area of the irregularities were more in the female runners than the male ones.
Macroscopic view of the surfaces of femoral articular cartilage in male control (A), female control (B), male runner (C) and female runner (D) groups. Smooth and glistening surface is observed in male and female control groups (A, B). However, the cartilage surface in male and especially in female runners become lusterless and rough (C, D) (notice the pointers).
3.2. Histology Observation in General
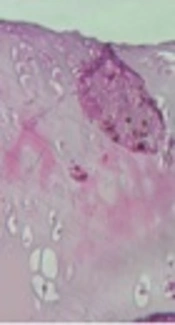
Normal histological features were observed only in male and female control groups. In contrast, histological changes of surface irregularities, cell cloning, and mild and moderate reduction in the H & E staining were observed in male and female runner groups, respectively (Figure 2).
Histological morphology of femoral articular cartilage in male control (A), female control (B), male runner (C) and female runner (D) groups; although grossly normal histological characteristics of cartilage sections are observed in male (A) and female (B) control groups, the osteoarthritic histological changes of surface irregularities, cell cloning, and moderate reduction are visible in male and female runners. H&E, 10X.
Results obtained by the Mankin method revealed mild OA both in male and female runner groups. It was noteworthy that the obtained results showed a significantly fewer damaged cases in male runner rats than the female ones. Obtained results are summarized in Table 1.
a, bMeans in a row without a common superscript are different (P < 0.05).
c0, no OA (osteoarthritis); 1-5, mild OA; 6-10, moderate OA; 11-14, severe OA.
d0-1, no synovitis (normal); 2-4, low - grade synovitis; 5-9, high - grade synovitis.
The synovial layer both in male and female control groups was normal. There were no multi - layer or inflammatory cells in the male and female control groups. Male subjects in runner group except four ones had a normal condition similar to the males in the control group. Other male subjects had just an increase in up to three layers. In addition, a moderate infiltration of plasma cells was observed in three of them (Figure 3). Based on the grading proposed by Krenn et al., the thickness of synovial layer in male runners was 0.8 ± 0.36 µm. There was no sinovitis in this group in comparison with the grading suggested by a previous study. On the other hand, in the female runner group, the synovium of joint appeared slightly coarse and was thick in all female subjects in the runner group, with a mild hyperemia dropsy display (Figure 3). In addition, based on the grading by Krenn et al., the thickness of synovial layer in female runner group was obtained 4 ± 0.42 µm. There was a moderate sinovitis in this group in comparison with the grading suggested by a previous study (20). The summary of obtained results in Mankin and synovial evaluation are presented in Table 1.
The synovial layer in male control (A), female control (B), male runner (C) and female runner (D) groups; this layer consists of a layer of cells with a smooth connective tissue in controls (A, B), while there is more than one layer in male and female runner groups (C, D). Arrows shows the synovial layer. H&E 40X.
Histomorphometric analyses were performed on H & E - stained sections in the four groups to obtain the cartilage thickness and chondrocyte number content. Although cartilage thickness decreased in the male runner group compared to the male controls, the difference was not significant. However, the cartilage thickness was significantly lower in the female runner group than the female controls (P < 0.05). Similar changing pattern was observed for a number of chondrocytes (Table 2).
a, b, cMeans within a row with different superscripts are significantly different (P < 0.05).
4. Discussion
The current study results showed that moderate running exercise could significantly affect the cartilage parameters such as cartilage and synovial layer thickness in female runner rats in comparison with those of the control group and male runner rats. These changes lead to OA occurrence in female runners more than male runners.
Recently, athletes and coaches show great interest in physiological tests to track changes in their training status, predicting their performance, and selecting proper training programs (20). Generally, physical loading is a double - edged sword. The proper running-induced joint loading remains undetermined (21). In the current study, it was suggested that moderate - intensity running damaged the articular cartilage, and therefore, it should be considered as a strenuous exercise, particularly for females.
In this regard, Ni et al., revealed the intensity - dependent impact of treadmill running on the lubricin metabolism of articular cartilage in rats (22). In another study, they showed different cartilage responses by changing the running intensity. Their findings showed that running load might be within and above the upper safe limit during running with low - to - medium and high intensities, respectively (15). Chondrocyte number and cartilage thickness reduced insignificantly and significantly in male and female runner groups, respectively, versus the controls. This finding was in line with the results of some previous studies. For instance, Ni et al., showed that high - intensity exercise reduced chondrocyte number, cartilage thickness, and glycosaminoglycan (GAG) level (23). Some studies also reported similar findings on beagle femoral cartilage following a running exercise (20 km/day or 40 km/day) during 15 weeks (24, 25).
In contrast, mild or moderate running improved cartilage GAG content in dogs (26) and humans (27). In other studies, cartilage integrity could be maintained via low - to - medium - intensity running. It was clearly demonstrated that chondrocyte count, cartilage thickness, GAG content, and collagen level significantly increased during running with low - to - medium intensity, suggesting some positive effects on cartilage integrity (9, 28, 29).
Moderate - intensity running may cause antiapoptotic effects on an intact cartilage and increase the chondrocyte count (7). However, the results of some previous studies on high - intensity exercise showed a decline in GAG content. In the current study, the loading on cartilage, induced by moderate - intensity running (which caused OA - like changes) exceeded the upper safe limit. The cartilage extracellular matrix may be damaged due to excessive mechanical stress, and the balance in chondrocytes is shifted in favor of catabolic activity over anabolism (30); these changes degrade both proteoglycans and collagen fibrils (31).
Gender differences in knee cartilage are examined in few studies. The obtained results revealed that females showed more cartilage changes in a moderate - intensity exercise, compared with males with the same body weight. Some studies indicated that differences in knee cartilage volume were significant in adults aged 26 - 61 years. A previous study, based on radiographic assessments, revealed that males’ cartilage volume were 33% - 42% larger than that of females in normal cases (7).
Faber et al., showed that gender differences did not significantly affect cartilage volume after body weight and height adjustments (13). Ding et al., specified that body and bone size had significant effects on this difference, which was in agreement with some speculations, suggesting that cartilage volume had a direct relationship with bone size (12, 32, 33); consequently, males have a thicker cartilage than females (3, 34, 35).
However, bone size differences were not adjusted in these studies. In contrast, another study found no gender - related effects after adjustments for the tibial head diameter and body weight (36). The current study showed some significant changes in rats with the same body weight and age. Therefore, other parameters may describe the gender differences. Nevertheless, these measures are highly reproducible and reflect the joint structure and surface. Therefore, growth factors and sex hormones are the most important factors to explain this difference (37).
4.1. Conclusion
Clearly, males and females should follow different exercise protocols. The current study results showed that the high exercises were more harmful in females than males. The current study results showed moderate treadmill exercise could lead to mild OA both in male and female rats, while histological changes in female runners were significantly more than male runners.