1. Background
Tuberculosis (TB) is a contagious and infectious disease caused by the Mycobacterium tuberculosis bacterium in humans. TB bacillus can be in a dormant state within the tissues of the body and can stay in the same position for years. Moreover, TB has the highest death rate among infectious single-agent diseases (even more than AIDS, malaria, and measles). The contagion source of the disease is a smear-positive pulmonary person with TB who diffuses droplets containing germs in the air around them by coughing and sneezing. Each cough can produce up to 3000 infectious particles. These particles can spread throughout the air by talking, sneezing, spitting, and singing. They can remain floating in the air for a long time and be inhaled by other people, enter their lungs, and cause contamination or infection if there is a favorable context in person (1).
The other transmission routes of the disease are skin contact during working with infected materials (ex: working in health centers), mother-to-child, and also through livestock products (ex: milk), but these ways are very rare and less important in comparison to transmission via coughing and sneezing of individuals with TB disease. Interestingly, pulmonary TB does not transmit via food, water, sexual contact, transfusions or insect-bites. Crowded indoor places without proper lighting or ventilation and humidity provide the best conditions and facilitate the transmission of the infection. These conditions can be found copiously in prisons, nursing homes, and shelters (1).
TB bacillus survives in the floating dust for about 8 to 10 days, in cool and shaded soil for 6 months, and in sputum for weeks or months; therefore, the air of an infected room with TB germs by infected patient can also transmit the germs even in the absence of the patient. Direct sunlight can destroy TB germs within 5 minutes; therefore, in the tropics, direct exposure to the sunlight is a proper way to destroy TB germs.
The disease is found in two forms in the human. First, pulmonary tuberculosis, constituting 80% of TB cases and it has two types: (1) smear-positive pulmonary tuberculosis due to the high risk of respiratory infection in people who are in contact with an infected person. It is of utmost importance in fighting against and controlling TB because if a smear-positive patient left untreated, he/she can infect 15 to 20 individuals during the first year. (2) smear-negative pulmonary tuberculosis that the risk of transmission is low.
Second, extra-pulmonary tuberculosis that includes the infection of other organs via the bloodstream, lymphatic vessels or proximal transmission from one organ to another. This form of the disease has a low risk of person to person transmission. TB disease has the tenth rank in the Global Burden of Disease, and is expected to move up to the seventh rank by 2020. According to statistics of the World Health Organization (WHO), each year nearly two million people lose their lives due to this disease. More than 80% of the infected persons belong to 22 developing countries. The incidence and prevalence of TB disease are high in Iran. Owing to the suitable factors and conditions for the survival of TB, Sistan and Baluchestan province has been the host of TB for a long time and despite the extensive and valuable efforts made by the healthcare system, it still has the highest number of TB incidence in the country (2, 3).
Recently, numerous studies have been conducted in related areas of research, including: Epidemiologic trend of smear-positive, smear-negative, extra pulmonary and relapse of TB in Iran (4); A comparison of before and after anti-tuberculous therapy (5); Survival rates among co-infected patients with human immunodeficiency virus/tuberculosis in Tehran (6); Tuberculosis incidence and its predictive factors among patients receiving antiretroviral therapy in Dilla Hospital, Ethiopia (7); Finding tuberculosis in prisons in India (8); Silico-tuberculosis and associated risk factors in the central provinces of Iran (9); The prevalence and associated factors of HIV infection among male prisoners in Tehran, Iran (10); Challenges of TB elimination (11); Semiparametric likelihood inference methods for left-truncated and right censored data (12); Cross-sectional studies of TB prevalence in Cambodia between 2002 and 2011 (13); Yield of pulmonary TB cases by symptoms: findings obtained from a community survey in Madhya Pradesh (14); High prevalence of pulmonary TB in household contacts with and without symptoms (15); Estimating the impact of TB anatomical classification on treatment outcomes (16); Evaluating treatment outcomes and durations among cases of smear-positive pulmonary TB in Yemen: a prospective follow-up study (17); Trend of TB case notification and treatment outcome in Lagos State (18); Cost of TB treatment: Evidence from Iran’s health system (19); Spatial-temporal distribution of smear-positive pulmonary tuberculosis in Liangshan Yi autonomous prefecture, Sichuan province (20); A comparative study of phenotypic and genotypic first- and second-line drug resistance testing of Mycobacterium tuberculosis (21); Trend of smear-positive pulmonary TB in Iran during 1995 - 2012 (22); Predicting the incidence of smear-positive TB cases in Iran using time series analysis (23).
Considering effective factors such as the high level of immigration from the affected surrounding countries (Afghanistan and Pakistan), poverty and its complications (malnutrition), addiction, poor health, increase in number of patients with AIDS in recent years, demographic and cultural situation of the region, there is a long path in the course of controlling TB. Because of the high outbreak of smear-positive pulmonary tuberculosis among other different forms tuberculosis, we studied this particular type of disease in this research.
2. Objectives
The purposes of this study is to evaluate the survival probability of patients with TB and their recovery time under incomplete observation with the condition of auxiliary variables of weight, which is effective on the TB disease, and the comparison between men and women, people with and without a history of the disease, whether the patient was in contact with someone involved with TB, whether the person had a history of imprisonment, and whether they had diabetes.
In order to achieve these aims, we used R software, Kaplan-Meier’s unconditional non-parametric survival probability and comprehensive (24), and software package of GTE (generalized Turnball’s estimator) for non-parametric conditional survival function (25). It is worth mentioning that owing to the particular circumstances of the data, regular analysis methods and SPSS software cannot perform the calculations of the survival probability.
3. Methods
This study was conducted with the purpose of determining the survival rate (possibility of staying alive) of patients with smear-positive pulmonary TB who referred to the Tuberculosis Coordinator Center of Zahedan, from 2014 to May 2016. Firstly, the information concerning the files of 250 patients with smear-positive pulmonary TB was collected and the diagnostic criterion for studied participants was a microscopic observation of the sputum. All patients had a chest X-ray and were examined by an infectious disease specialist and smear-positive pulmonary TB and they were diagnosed with the disease.
It should be noted that the confidentiality of patients’ information is an ethical consideration of this study. In this study, we need main and auxiliary variables to analyze the data; therefore, we examined the main and auxiliary studied variables as the following: survival time for people with TB disease was the main variable, which might depend on auxiliary variables such as patient’s weight at the time of diagnosis, blood type, race, sex, nationality, HIV infection, the presence of diabetes, history of being in contact with a patient with TB disease, a history of previous infection with TB disease, a history of attendance in a prison or a nursing home. The existence of some auxiliary variables is effective in the treatment and recovery of the disease or in resistance to the disease, which are considered effective auxiliary variables. In this study, we have considered the patient’s weight as the auxiliary variable.
3.1. Survival Time Description
In this study, the interval between the beginning of treatment and discontinuation of treatment is considered to be the study period for each patient in which we faced two types of censorship:
1. People who left the treatment in this interval for any reasons except for death, change in location, etc. and we have no information about their future, we called them right censored at the last positive appointment (the existence of the disease).
2. For individuals who have recovered, we considered the last positive appointment Li (the existence of the disease) and the first negative result Ri (non-existence of the disease) as interval censored and we showed it with (Li, Ri; i: 1, …, 250).
In survival studies, if we have accurate data and right censored, generally Kaplan-Meier estimator will be used to estimate the survival function. If data includes accurate data, interval censored, right censored, this estimator cannot calculate survival function; as a result, a new estimator called non-parametric estimator of survival probability will be used, which is presented by Dehghan and Duchesne (GTE) (25, 26). This estimator uses the auxiliary variable and core function to increase accuracy more accurate than the Kaplan-Meier estimator and Turnbull’s unconditional estimator, and it is suitable for a variety of accurate and censored data or a mixture of both. In addition, we can observe the effect of different variables on the survival probability estimator and focus our attention on specific points of the auxiliary variable. Mentioned estimators of non-parametric conditional survival probability are defined as follows: T is the time to recovery (that can be measured in days, weeks, months, etc.), the Z is an auxiliary variable. In the current study, time is measured in days and the auxiliary variable of weight is measured in kilograms (26). The K(.), is the Gaussian Kernel (normal core) function with bandwidth h that is chosen based on the (27), used in the survival probability estimation (27).
4. Results
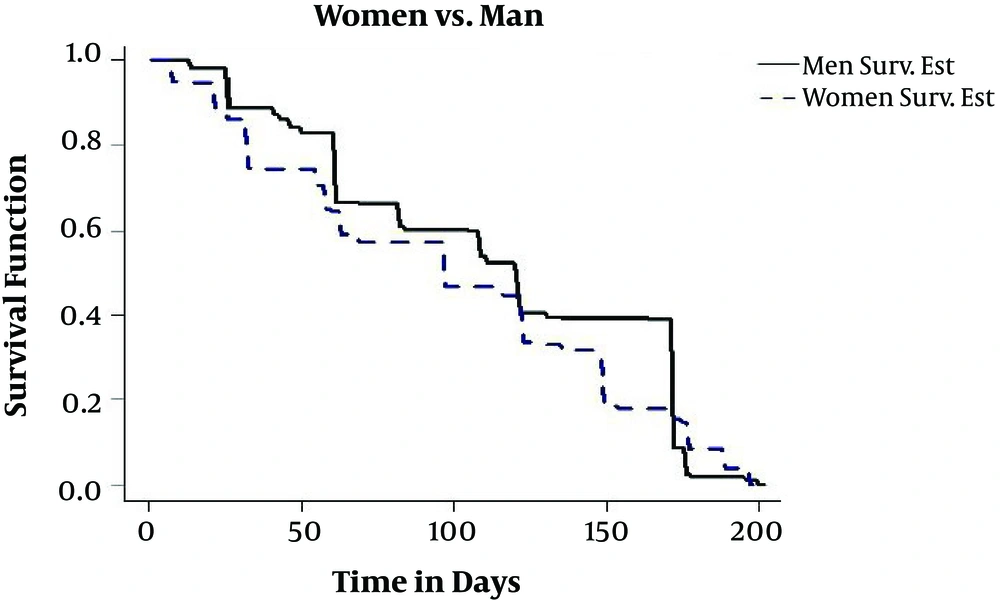
The number of studied patients was 250, who had files in the Tuberculosis Coordinator Center of Zahedan. Four patients are right censored and the rest are interval censored. Patients’ age is from 10 to 91, and their diagnosis time is between 2014 and 2015 and have recovered by May 2016. The average age of diagnosis among infected males is 58.54 with a standard deviation (SD) of 19.10 years and among infected females, it is 55.40 with an SD of 18.09 years. Among children up to the age of two, the risk of more deadly forms of outbreaks such as TB and TB-related meningitis is higher. In this period, there are no major differences between females and males, but females after puberty, pregnancy, and childbirth are more likely to catch TB than men. According to the studies carried out in Iran, it seems that the outbreak of TB is more likely in people older than 50 years of age (17) and the results of this study also confirmed it. In this study, most cases of the disease are in the 50 - 60 age group, it should be noted that in this age group, the body is less immunocompetent and individuals can come down with an illness faster. In terms of transmission within the sex groups, 49.2 percent of infected people were males and 50.8 percent of the patients were female. Also, in the study of Shoraka et al. in Northern Khorasan 54.1% of patients were females (28). Furthermore, in the study of Sofian et al. (29). In Arak 61.9% of patients were female and also in the study of Heshmati et al. 55.1 percent of patients were female (2). In this study, for estimating conditional survival function, GTE software package was used. In Figure 1, we have drawn the estimation of conditional survival probability between the groups of males and females. As you see, the female’s group has lower resistance than the male’s and the recovery possibility is greater in males than females. One of the reasons that resistance in a female’s body is weaker than a male’s body is childbirth and consecutive deliveries, which reduces the immunity of the body. It should be noted that TB is the most important cause of female’s fatality in the world. It even has a higher fatality rate than factors related to pregnancy and childbirth. Besides, female’s infection to TB can have a major impact on TB infection of the family, because mothers are at home for more hours to take care of children and other family members and they have more opportunity to transfer the disease to family members. Moreover, in developing countries, where the mother plays an important role in the family economy, when the mother gets sick, it also causes economic problems for the family. It should be noted that the rate of females’ infection, in all studied age groups, has been more than male’s.
Owing to the high rate of women’s TB infection, it is suggested that in areas with large amounts of TB outbreaks, taking action to build sunlit homes and schools in order to use the sunlight to destroy the germs should be considered.
Annually in Afghanistan, 60 thousand people come down with TB, in which about fourteen hundred people lose their lives because TB is at a critical phase in Pakistan. In this study, 33.6% of patients were non-Iranians because of the proximity of the province with the two countries mentioned above. In order to control the outbreak in the province, it is suggested to set up an appropriate quarantine zone at the country’s ports of entry in order to test people’s health and prevent sick people’s entrance.
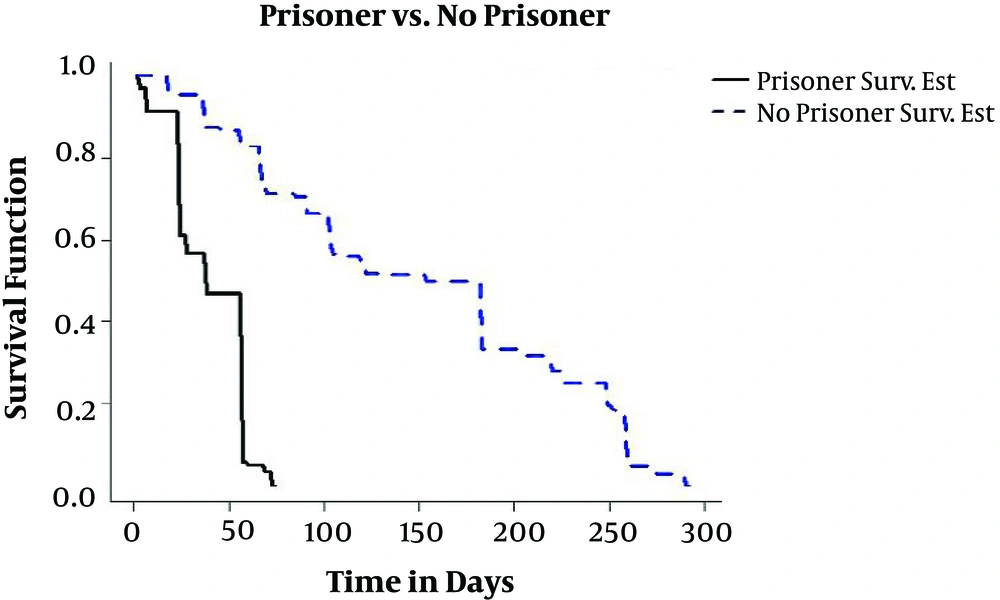
In this study, 17.6% of patients had a history of imprisonment. In Sofian’s study 1% (29) and the study of Heshmati et al., 7.9% (2) of patients had a history of imprisonment. Figure 2 shows the estimation of conditional survival probability between individuals whether having a history of imprisonment. As we can see, non-prisoner patients have bodies with higher resistance, in comparison to prisoner patients, and have a higher chance of recovery. In global conducted studies, it has been shown that TB outbreak rates in prisons are one-hundred times higher than its rate in a normal society; because the lack of a healthy space, ventilation, and abiding by the personal hygiene extremely facilitate the transmission of the diseases; thus prisons are a source of TB.
On the other hand, the high rate of TB in prisons has played a significant role in spreading the disease in society.
The high density of prison population in comparison to prisons and also return of released prisoners to the society along with predisposing factors, such as lack of commitment to health conditions and health tracking, all collaborated to cause TB outbreaks. Sanitary imitations within prisons and their consequences on the outside make prisons a challenge in the control of TB in all countries, specifically in developing countries. In this study, 1.2% of individuals, in addition to TB, are AIDS-infected. Owing to the weakening of the immune system in an AIDS-infected person and TB bacteria resistance to antibiotics, in comparison to other bacteria, it becomes opportunistic and is activated inside the AIDS-infected person’s body and accelerates TB disease. It should be noted that in Center for Global Health’s report, more than 50 percent of people who had already been infected with TB and were later infected with the AIDS virus, the TB disease creates ideal conditions for the contraction and activation of AIDS (10). This complication is of particular more common among people who inject drugs. Diseases such as TB and syphilis are sources of AIDS outbreak among individuals, and those who are infected with these two diseases are more prone to be infected with AIDS. In Iran, infection with TB is the most common cause of fatality of AIDS-infected patients. Thirty percent of people with AIDS who have lost their lives, had been suffering from TB (1).
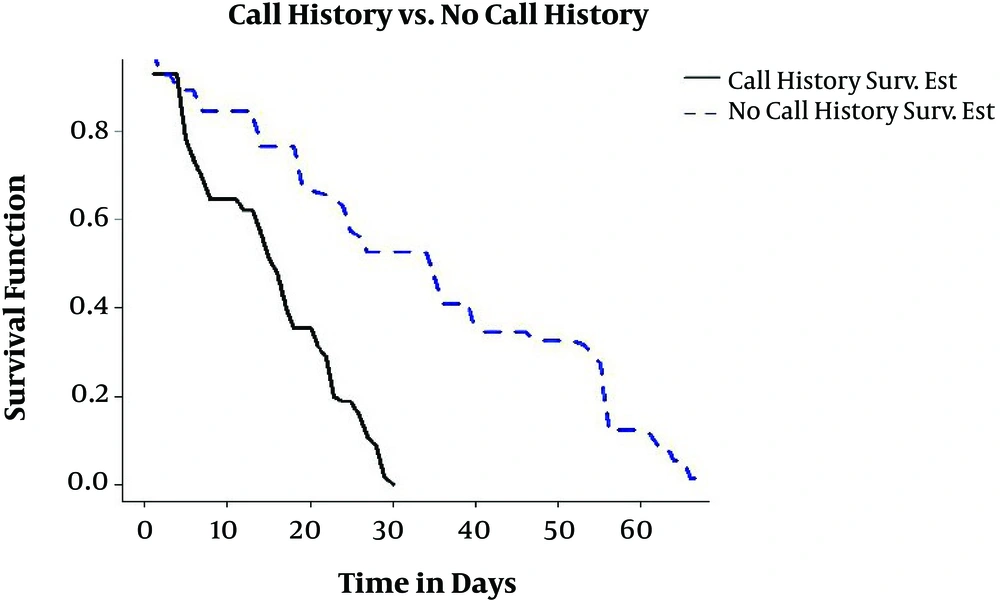
Moreover, 29.2% of individuals studied in this research were in contact with someone infected with TB; as previously stated, the main reason for transmission of TB is person to person transmission. In Figure 3, the estimation of conditional survival probability of patients who were in contact with someone infected with TB and patients who were not, is shown. As you can see, individuals not in contact have a higher chance of recovery. The treatment of a patient with TB is a secondary prevention measure for him/herself and primary prevention measures for other people of society. Each patient with TB can infect 12 people each year (3).
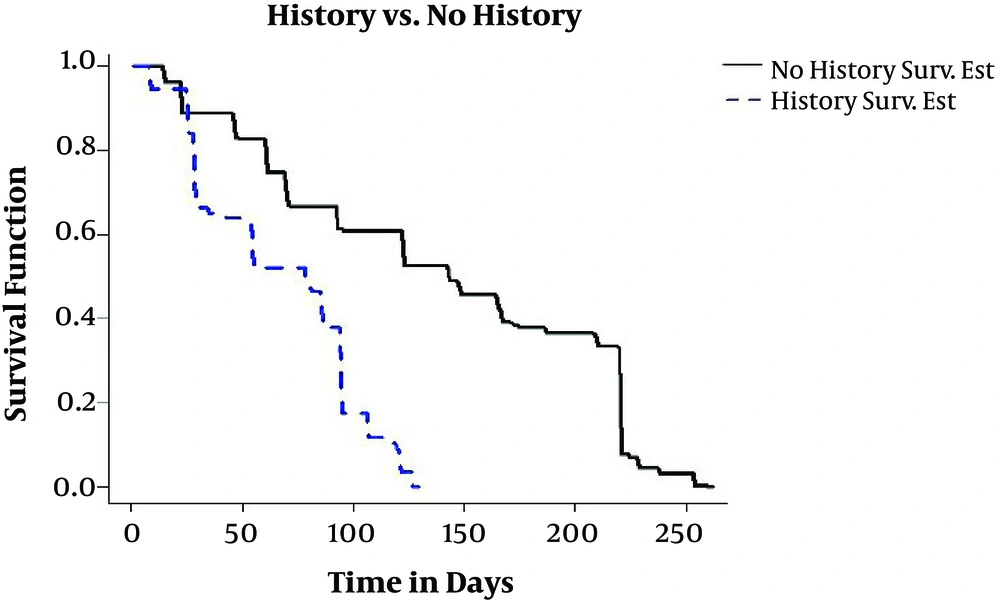
In this study, 21.2% of studied individuals had a previous history of TB. Individuals who were previously infected with TB and were confirmed by a specialist after the treatment and full recovery; they were re-infected after a few years. Therefore, they are at high risk in comparison to other people in society because of a reduction in the immunity of their bodies. In Figure 4, we can see a previous history of TB that has a negative impact on the treatment process in comparison to patients with TB who do not have a previous history because the bodies of TB-infected people with a previous history show less resistance to the disease.
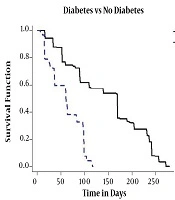
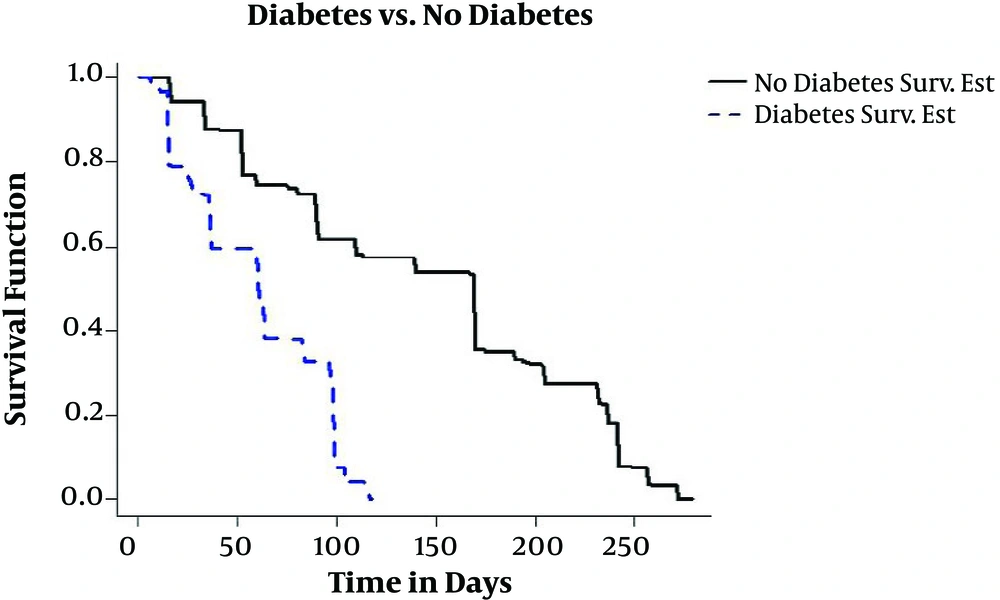
Since 1393, TB centers of the province were obliged to test TB patients for HIV as well as diabetes, which in this study 26% of the patients had diabetes. In Figure 5, the estimation of conditional survival probability of TB patients with diabetes and TB patients without diabetes is drawn. Owing to the reduction of the immune system of the body caused by diabetes, as it is expected, co-infection of diabetes and TB leads to negative results in the progression of the treatment in patients with TB and diabetes simultaneously in comparison to patients without diabetes.
In this study, in examining survival probability charts, the effects of the sex, history of co-infection with diabetes, previous history of having the disease, being in contact with a patient with TB disease, the patient’s history of imprisonment, are observable in the recovery time. In addition, we consider it necessary to examine the effects of these factors on the recovery time and the survival probability by using R to calculate the following table such as Independent Sample t-Test. Therefore, the effects of each of these factors on the survival probability are considered versus the control group (without the effect of the relevant factor) and Independent Sample t-Test is performed. As you can see in the Table 1, regarding the P value in all cases (it is less than 0.001), the result of the test indicates that the above factors are highly significant in the survival probability (recovery time) of the patients.
| Subjects | Mean ± SD | P Value |
|---|---|---|
| 1 | 2.2e-16 < 0.001 | |
| Male | 53.55 ± 11.34 | |
| Female | 51.19 ± 11.94 | |
| 2 | 3.16e-7 < 0.001 | |
| Diabetic | 52.78 ± 11.38 | |
| Non-diabetic | 52.18 ± 11.83 | |
| 3 | 2.2e-16 < 0.001 | |
| History of the disease | 52.32 ± 11.97 | |
| No history of the disease | 52.34 ± 11.61 | |
| 4 | 2.2e-16 < 0.001 | |
| Contact with TB patient | 52.22 ± 11.61 | |
| No contact with TB patient | 52.65 ± 11.98 | |
| 5 | 2.2e-16 < 0.001 | |
| History of being imprisoned | 52.09 ± 12.05 | |
| No history of being imprisoned | 53.65 ± 9.62 |
5. Discussion
In this study, regarding censured data, the regular conditional survival analysis methods are not possibly applicable. Therefore, we used the GTE (25) method and the patients’ weight was considered a condition and effective auxiliary variable in the evaluation of the survival probability. Also, we considered the effect of factors such as sex, co-infection with diabetes and/or AIDS, previous history of being in contact with TB-infected patients, and a history of imprisonment on the survival function. The findings of this study revealed that all of the mentioned co-factors are significantly effective in the course of the treatment, the patient’s recovery time, and the survival time.
In this regard (5), they conclude that CT shows the development of tuberculosis in diabetic patients differing from those in non-diabetic patients just. Diabetes negatively impacts the treatment outcome, and the resolution of lesions on CT in patients with diabetes is slower than that non-diabetic patients. CT can provide vital information considering the diagnosis and management of the disease. This is particularly helpful in the early stages of the treatment because only half of the patients with TB show acid fast-positive bacilli in their sputum. In addition, Roshanaei et al. (6), reported a set of modifiable and non-modifiable high-risk factors affecting the mortality of patients co-infected with just HIV/TB. The current research recommends an interplay between the mentioned risk factors and the risk of death in co-infected patients with HIV/TB.
Obviously, they considered thus study with precise observations. But we considered conditional survival probability (with various factors) under censored data, meaning a mix of right and interval censored observations. According to the study’s results, the most important prevention factor of TB disease is teaching the community about this disease that is suitable for all schools, especially in girls’ schools, to hold health education classes about TB prevention for all students. Owing to the role of religious centers in the lives of people of this province, we can use the collaboration of these centers with health centers to advance the prevention of this disease Infected patients, suspected of having TB, must be identified and perform a sputum test. Follow-up is necessary in case of the existence of the disease. There must be consultation meetings for individuals in close contact with a person with TB. Sputum tests must be taken in prisons at their arrival and education classes must be held.