1. Background
Male sexual dysfunction is associated with problems such as low sperm concentration and motility and hormonal imbalances. These conditions can be due to toxic chemicals, smoking, alcohol consumption, drug abuse, anti-depressant medicines, and senescence. CCl4 is known as an industrial solvent (1). Carbon tetrachloride is an industrial solvent that has been found to cause liver, kidney, lung, and testicular damages in experimental animals (1, 2). In fact, CCl4 is well known for a hepatotoxin agent, which causes steatosis, necrosis, and cirrhosis in animals (3). Some studies indicated that in hepatic cirrhosis, which induced by carbon tetrachloride, due to reduction of transferrin expressed by sertoli cells, spermatogenesis was impaired. Some cytokines in the testicles, such as IGF-I (Insulin-like growth factor-I), are reduced in cirrhotic rats. This cytokines decreases the cirrhotic effects in the testicle (4, 5).
The metabolism of carbon tetrachloride leads to the release of free radicals, that by binding to polyunsaturated fatty acids (PUFA) of the plasma membranes of sperms, results in production of alkoxy and peroxy radicals, where in turn, produce highly reactive lipid peroxides that can affect sperm numbers, levels of hormones, enzymes activity, and even necrosis (2). Spermatogenesis in mammals testis takes place in the convoluted seminiferous tubules (6). Spermatogenesis is transitioning from mitotically active primordial germ cells to mature spermatozoa and it involves a sweeping series of structural transformations. The overall process of spermatogenesis is the same throughout the vertebrate classes. This process can be divided into three major phases: (1) Proliferation by mitosis, (2) meiosis, and (3) spermiogenesis (7). Studies have been shown that carbon tetrachloride was able to induce destruction in seminiferous tubules and alteration in the spermatogenic cycle in rats (3). Herbal medicine or herbalism has been used for treatment of a wide variety of diseases from the past decades, due to the fact that they are more available, inexpensive, and more effective. They are exerted to prevention of diseases associated with oxidative stress (8). A wide range of phytochemical molecules with antioxidant effects, including carotenoids and phenolic compounds are present in these plants (9). There are similarity in antioxidant properties of many plant species without any side effects. These plants are used in the food processing industry and preventive medicine as a valuable alternative (8). The subgenera of the genus Sophora (Fabaceae) are Sophora and Styphnolobium. The genus Sophora is consisted of 52 species around the world from which, three species have been identified in Iran, including S. mollis 2, S. alopecuroides L, S. pachycarpa, and a hybrid S. alopecroidese × S. pachycarpa (10). S. pachycarpa is a herbaceous perennial, 30 - 60 cm tall (11). In several studies, chemical composition of S. pachycarpa has been analyzed and the results showed the presence of quinolizidine alkaloids, steroid glucosides, and flavonoids. The alkaloids of the different species of Sophora have been known to be the major (12). Sophora sp. alkaloids have been known to be the chief active compounds such as sophoridine, sophoramine, sophocarpine, oxymatrine, and matrine. Clinical and basic investigations revealed that these types of alkaloids have a wide variety of pharmacological effects including anti-inflammatory (13, 14), immunity-regulatory (15), antiviral (16), antibacterial (17), and anti-tumor properties (18, 19). Therefore, it would be important and applied to confirm the antioxidant properties of Sophora pachycarpa. For this purpose, the present study was undertaken to evaluate the protective effects of the Sophora pachycarpa extract on testicular histopathology in carbon tetrachloride-intoxicated in male rats.
2. Methods
A voucher specimen (NO. 06-019-016) is entrusted in herbarium of the School of Pharmacy, Herbarium of the Faculty of Pharmacy, Mashhad University of Medical Sciences, Mashhad, Iran. The roots of plant were dried and then pervaded with methanol at room temperature. The whole extract was filtered and the solvent was evaporated under reduced pressure at 40 - 450°C to afford crude methanolic extract. Then, the collected extract dissolved distilled water and KCl to prepared required dosages.
Male albino rats of Wistar strain (195 - 200 g) were purchased from Tabriz University of Medical Sciences (Tabriz, Iran). The animals were kept in cages made by polypropylene in a temperature-controlled room (22 ± 20°C) with 40% - 60% relative humidity under cycles of light and dark (12/12 h) for 1 week prior to and during the experiments. The rats had standard laboratory feed (pellets) and drinking water. Treatment of rats with CCl4 was performed according to Eshaghi et al. (20), which some modifications have been made. Usage doses have been determined according to previous studies and results from them. In addition, the doses used were interpreted according to BSA formula (21, 22). Most studies have been done on the protective effect of plant extracts on carbon tetrachloride- induced toxicity has been used in mediocre doses.
Animals were grouped as 7 experimental groups, with 6 animals in each of them as:
Group I, II and III used as pre-treatment groups. They received SPRE at doses of 50, 100, and 250 mg/kg orally for 21 days, respectively, and on the day of 21 they received CCl4 (500 µL/kg body weight: ip), 3 - 4 hours after the last dose administration of the extract.
Group IV considered as control and 1 mL distilled water for 21 days.
CCl4 group receiving 500 µL/kg CCl4 on the day of 21.
Group VI served as post-treatment groups and received 250 µL/kg CCl4 on the 21st day followed by SPRE at doses of 100 mg/kg (orally) to groups VI for 10 days.
For microscopic evaluation, testis were fixed in a fixative (formalin 10%) for 48 h and stained with differential haematoxylin-eosin and were observed using a light microscope.
3. Results
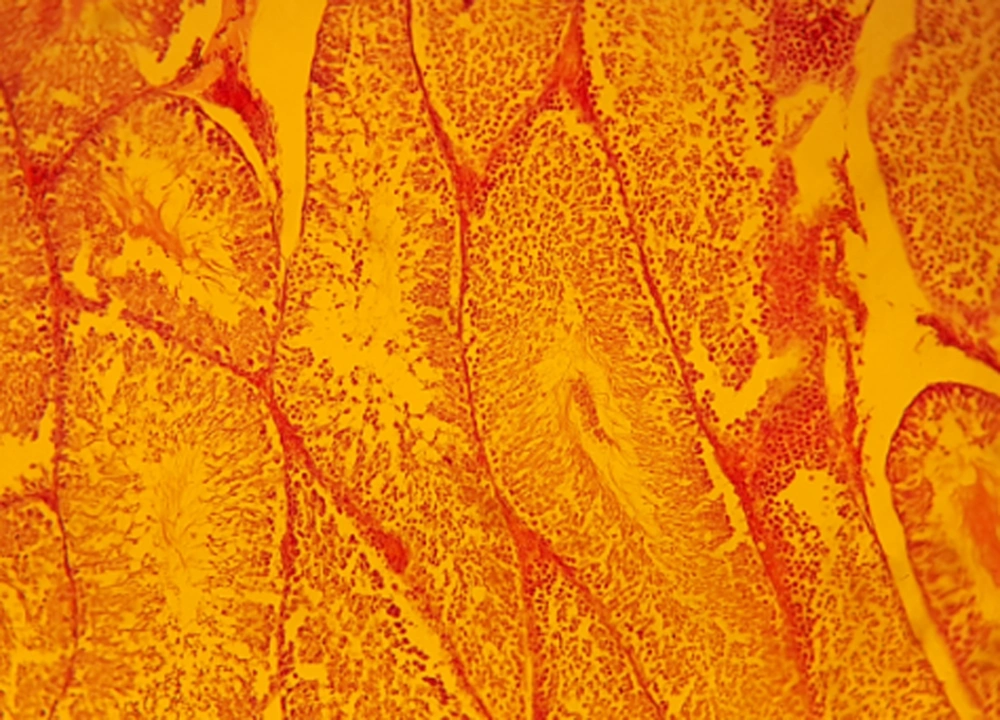
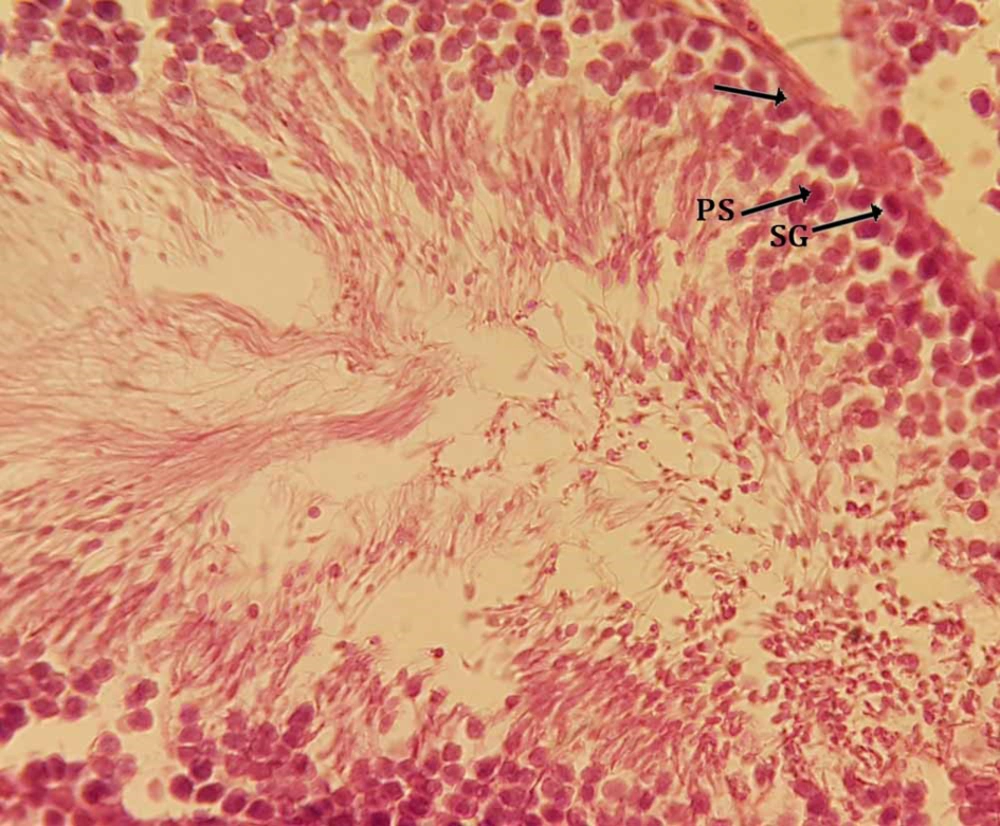
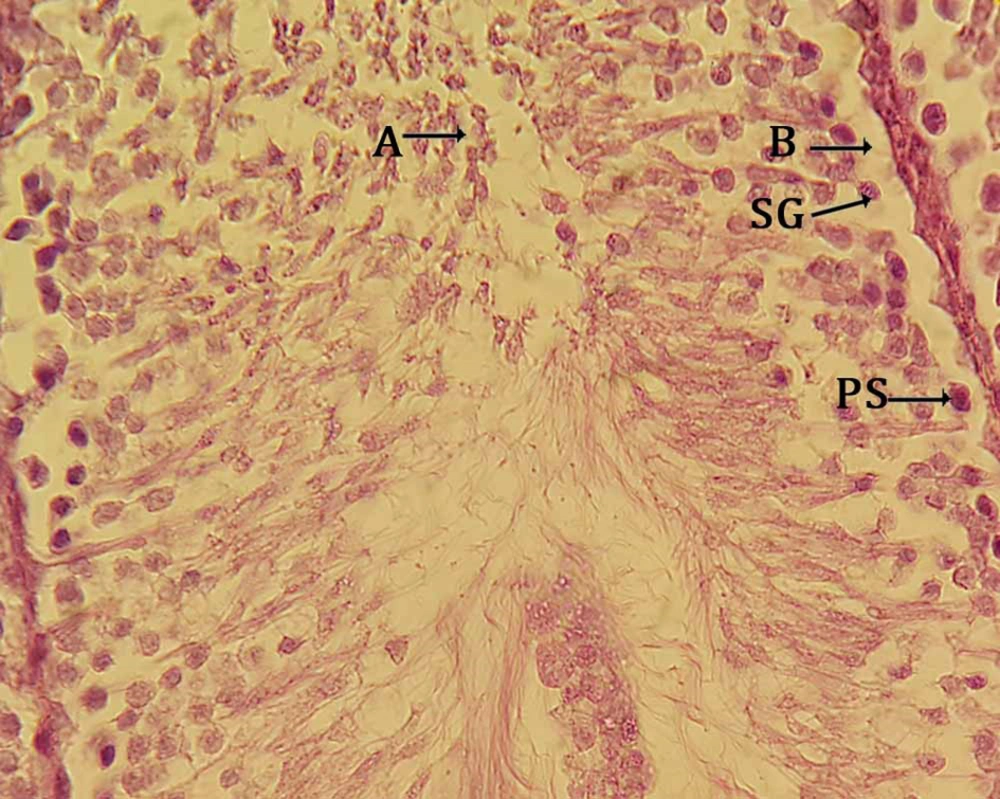
Sections in carbon tetrachloride treated group (experimental group 5) showed that the spermatogenic cells are limited to the lumen of the seminiferous tubular basement membrane. CCl4 administration caused degenerative changes in germinative epithelium of seminiferous tubules, loss of germ cells, meiosis division discontinuity, and decrease of sperm counts with their abnormal shapes. There were testis histology, atrophic changes of seminiferous tubules were exhibited while in other parts of the sections the basement membranes of seminiferous tubules were observed, however, most of the germ cells showed degeneration in Figure 1. In the (CCl4 + SPRE)-treated groups, especially at the higher dose of SPRE (250 mg/kg), toxic effects were clearly ameliorated, depending on the administered dose. It has been proposed that the dose of 250 mg/kg SPRE can be considered as an effective dosage in protecting from testicular toxicity-induced CCl4 (Figure 2). In pre-treatment groups I and II (50, 100 mg/kg) histopathological changes did not improve compared to Group III. In addition, in the post-treatment group, group histopathological changes were less moderated in comparison with the pre-treatment group and it can be said that the tissue sections were almost the same as the CCl4 group.
This study showed that the SPRE, in the doses used, did not have a toxic effect with carbon tetrachloride, however, the toxic effect of this extract only has not been determined on testicular tissue and we will determine toxic dose of SPRE.
4. Discussion
Infertility is considered as one of the major problems of human societies (23). According to the reports of the World Health Organization (WHO), 10% - 15% of young couples have suffered from infertility problems, 40% them were being due to male factors (24).
Male infertility may be caused by different factors including genetic abnormalities, obstructions in genital ducts, varicocele, decrease of sperm production, decrease of seminal fluid quality, erectile dysfunction, and being male.
Various treatment protocols are available to help fertility such as surgery, chemical agents, laboratory methods, and medicinal plants. Based on the findings of recent studies, some medicinal plants can improve sperm parameters (24). Due to the fact that effective produces was indicated in medicinal plants associate with other material, they have a biological balance and don’t accumulate in the body, therefore, they do not cause side effects. Hence, they have a remarkable superiority over chemical drugs (25). ROS is suggested as one of the important causes of infertility in males, that is, 30% - 40 % of infertile men have enhanced levels of ROS in their seminal fluid plasma (26). In mammalian spermatogenic cells, specific structure of sperm plasma membrane, which is rich in polyunsaturated fatty acids, plasmalogenes, and sphyngomyelins, may also predispose to lipid peroxidation and ultimately, oxidative stress (27). In addition, the cells in this tissue are divided frequently and increase the production of free radicals highly. Decrease in antioxidant defense is also leading to severe accumulation of free radicals. Free radicals are produced from different pathways within the cell and caused tissue damage and apoptosis in the testis (28). In some previous researches on analyzing the chemical composition of S. pachycarpa, the presence of alkaloids and flavonoids was found (12). Flavonoids inhibit enzymes are involved in ROS production, which includes monooxygenases, glutathione transferase -s, sukcynooxidase, and NADH oxidase. Flavonoids protect lipids against damage of oxidative stress (29).
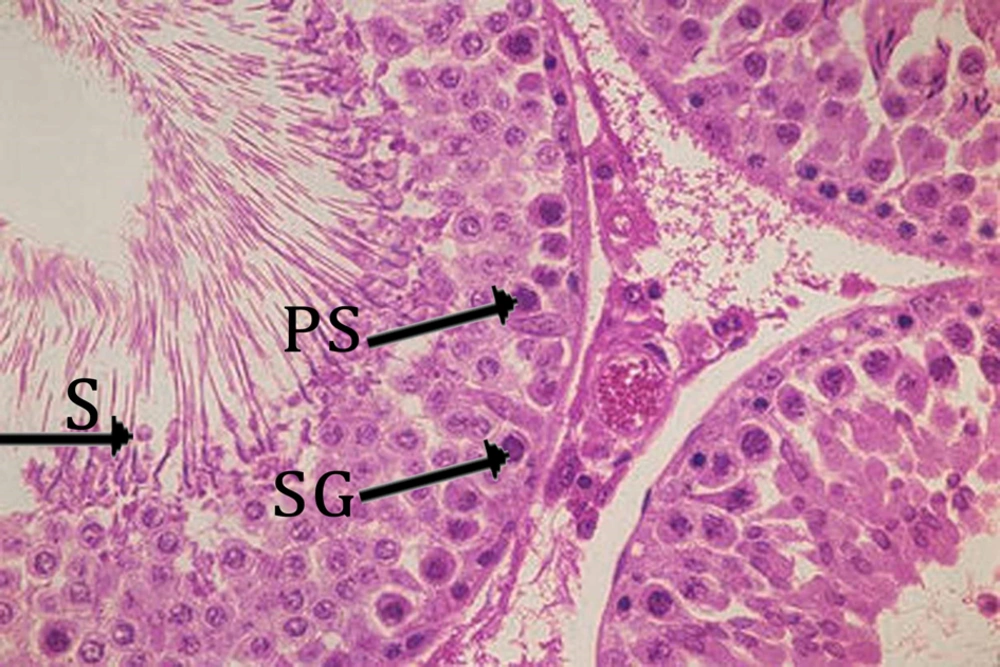
In this study, the adverse toxic effects of CCl4 on testis were observed. Histopathological examination in this study showed that carbon tetrachloride lead to changes, which include atrophy of seminiferous tubules, irregularity, and disruption of connections in germ cells, especially between the control group (Figure 3) compared with the spermatogonia and primary spermatocytes (Figure 4). The presence of flavonoids in S. pachycarpa may ameliorates the toxic effects of CCl4 (3). In addition, almost normal histological features was observed in SPRE treated groups. It was indicated that SPRE has a synergistic effect in hepatotoxicity with CCl4 due to the fact that S. pachycarpa extract in pre-treatment groups amplified the toxic effects of CCl4 (30).
In this regard, studies have been conducted similar to the present study. Dolatabadi and Khaki determined a protective effect of Cannabis sativa L extract on the reproductive system intoxicated in male rats. Study in testicular histomorphology showed that CCl4- intoxication lead to testicular atrophy and epithelium degeneration. CCl4 may inhibit the proliferation of group of germ cells and caused reproductive disorders. In the CCl4-treated group Leydig and germ cells degeneration have been shown. In addition, spermatogenesis is deformed. However, in the group that received the plant extract, spermatogenesis was well done in most parts and the seminiferous tubules basement membranes were innate (31).
Al-Olyana et al. studied the protective effect of pomegranate juice extract on testis against the toxic of carbon tetrachloride in male rats, which in CCl4-treated groups, germ cells showed degeneration, and interstitium almost disappeared and replaced by fibroblasts and inflammatory cells in some areas of testicles. It is believed that the presence of some compounds like saponins and flavonoids in pomegranate juice extract might be involved in improving the toxic effects of CCl4-induced toxicity and concomitantly almost normal histological features seen in pomegranate juice extract treated groups (1).
Study in the protective effect of Lactuca sativa on CCl4-intoxicated in rats showed that in post-treatment groups, Leydig and sertoli cells were intact, in addition, normal basement membrane of seminiferous tubules and interstitium was observed. However, in CCl4-treated group complete atrophy of seminiferous tubules have been seen. Furthermore, normal basement membrane in some areas of tubule were identified while germ cells showed degeneration. Interstitial tissue disappeared and was replaced by inflammatory and fibroblast cells (32). The study of Eshaghi et al. showed injection of 1 mL/kg of carbon tetrachloride-induced testicular atrophy and death of spermatogenic cells in comparison with the control group, however, receiving the Cornus mas corn extract before treatment with CCl4 could improve the histopathological changes compared to CCl4 group (20). Investigation in increase of glutathione, testosterone, and antioxidant effects of Jurenia dolomiaea on CCl4 induced testicular toxicity in rats revealed that treatment with 1 mL/kg of CCl4 resulted in dissociation, displacement, and alteration in shapes of seminiferous tubules. Vacuolization of germinal epithelium and prominent decrease in germ cells was also represented. In the administration of Jurenia dolomiaea, only at higher dose (400 mg/kg), reversed toxic effects-induced CCl4 (33).
4.1. Conclusion
This study indicated that being exposed to CCl4 lead to destructive effects on testis tissue and in general, the male reproductive system. CCl4 caused histopathological changes (loss spermatogenic cells into the lumen, cells disarray, etc.). The use of Sophora pachycarpa root extract before CCl4-intoxicated could ameliorated toxic effects, which is due to extract antioxidant properties. Therefore, it can be recommended to reduce the damage that is caused by free radicals, is suggested the people who exposed to these pesticides, which enhance their antioxidant power by use of natural antioxidants, however, recognizing the accurate mechanism of these materials in different toxicities require molecular studies in the future.